General Questions and Answers

Physical Chemistry
GeneralAt 25 C Ksp for PbBr2 is equal to 8 x 10 If the salt is 80 dissociated what is the solubility of PbBr in mol litre 1 3 1 1 60 2 3 4 10 4 1 6 1 6 10 5 1 6 1 6 10 4 71 3 0 8 0 8 71 3 10 5 1 6 1 6 71 2

Physical Chemistry
GeneralWhen an immiscible liquid A was steam distilled with water it gave 200 ml of distillate which contained 55 mL of A The boiling point for distillation was found to be 98 C at a pressure of 755 torr At this temperature the vapour pressure of water was 700 torr The density of the liquid A is 2 g mL Select the correct statement s Assume density of water is 1 g ml Vapour pressure of pure A PA at this temperature is 55 torr Mass of A in distillate is less than mass of water Mass of water in distillate is 145 g Molar mass of A is 173 8 g mol approx

Physical Chemistry
General63 At certain temperature compound AB2 g dissociates according to the reaction 2AB g B g 2AB g With degree of dissociation a which is small compared with unity the expression of Kp in terms of a and initial pressure P is 3 Pa b 3 a P 2 3 pa 3 c P d 2 Pa 2

Physical Chemistry
GeneralOFFICE OF CHIEF W All the students of 1 II III IV year who are staying in the hostels are advised to go to the class on time During the class hours if students are present in the hostel and not attending the class college management will take appropriate action This has the consent of Principal Jandl Dr Jagadish R S Chief Warden CC to Principal CAO AO Registrar Deans All HODs TPO Hostel Superintendents caretakers Parents Notice Board
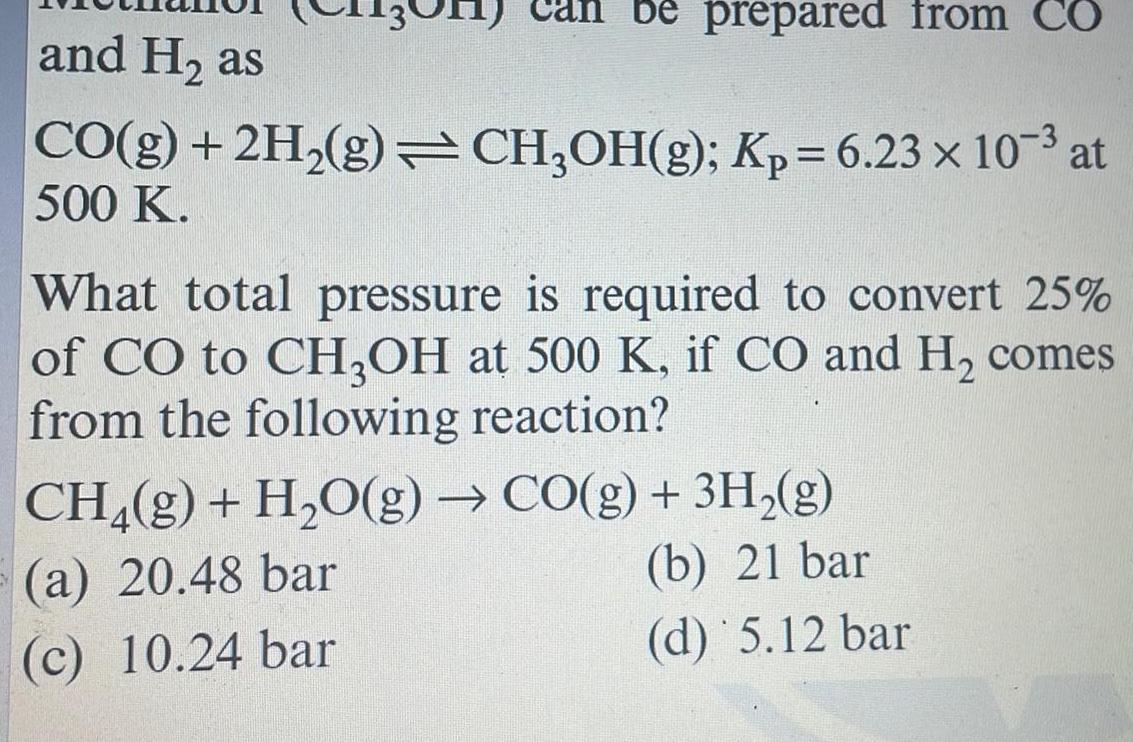
Physical Chemistry
Generalbe prepared from CO and H as CO g 2H g CH OH g Kp 6 23 x 10 at 500 K What total pressure is required to convert 25 of CO to CH3OH at 500 K if CO and H comes from the following reaction CH g H O g CO g 3H g a 20 48 bar b 21 bar c 10 24 bar d 5 12 bar

Physical Chemistry
General5 If Dr and Do are the theoretical and observed vapour densities at a definite temperature and a be the degree of dissociation of a substance then a in the terms of Do Dr and n number of moles of products formed from 1 mole reactant is calculated by the formula DT Do DT Do n 1 DT n 1 Do a a Do DT 1 n DT b a c a d a H D DT n 1 Dr

Physical Chemistry
GeneralAll of the following statements are correct regarding potentiometric titrations except O O O O Liquid junction potentials will not influence the study The emf of the cell is zero at the equivalence point They are suitable for colored or turbid reactions These are not suitable for analysis of dilute solutions less than 0 001 M

Physical Chemistry
GeneralSuppose 12 9 g of lead II acetate is dissolved in 150 mL of a 0 50 Maqueous solution of ammonium sulfate Calculate the final molarity of lead II cation in the solution You can assume the volume of the solution doesn t change when the lead II acetate is dissolved in it Be sure your answer has the correct number of significant digits M 10

Physical Chemistry
GeneralRepeat 10 00 mL aliquots of a CAM solution with a molarity of 0 04 were titrated using a NaOH solution The mean titration volume of the NaOH solution for an acceptable set of titrations was 12 26 mL Calculate the molarity of the NaOH solution State the answer to four decimal places and without units

Physical Chemistry
General10 For the following mixture of oils ver Cottonseed oil Cetyl alcohol 2 0 g Calculate RHLB and the quantity of surfactants to prepare 100 mL of an or emulsion RHLB 10 5 18 0 g Use a mixture of Span 20 HLB 8 6 and Tween 60 HLB 14 9 This mixtur should amount to 2 of the formula Span 20 1 4g Tween 60 0 6g
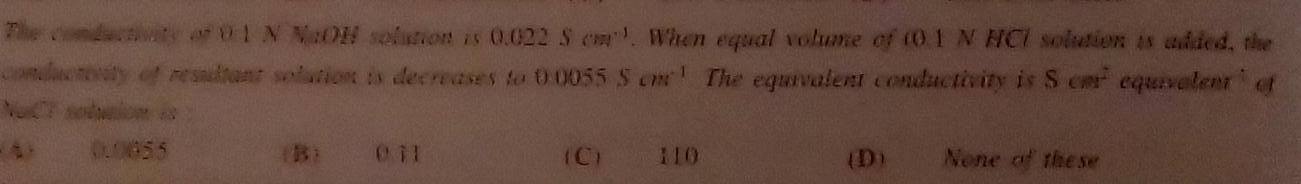
Physical Chemistry
GeneralThe conductivity of 01 N NaOH solution is 0 022 S When equal volume of 0 1 N HCI solution is added the conduceaty of resultant solution is decreases to 00055 S cm The equivalent conductivity is Scar equivalent of 00855 Bi 011 None of the se

Physical Chemistry
GeneralPhase sensitive AC polarography technique depends on the following fact O O O O Voltage Lags Current by 90 Capacitive Current lags Voltage by 90 O Faradic Current lags Voltage by 90 Faradic Current lags Capacitive

Physical Chemistry
General2CO g CO g C s This question asks to calculate the equilibrium k of the reaction at 800 C and 2 bar in additi to the degree of advancement of the reaction in equilibrium and the partial pressures of CO at CO2 in equilibrium

Physical Chemistry
Generalbases are sufficiently strong to deprotonate a termin alkyne pka Select all apply Points will be deducted for incorrect answers NaH pKa of H 35 NaOCH3 pK of CH3OH 16 LIOH PK of H O 15 7 H O pK of H3O 1 7 CH3Li pK of CH4 60 KOC CH3 3 PK of CH3 3COH 18 n BuLi pK of CH3CH CH CH3 50 NaNH pK of Hy 36 3

Physical Chemistry
General1 386 g of p aminophenol MW 109 13 g mol is reacted with 2 926 g of acetic anhydride MW102 09 g mol to form acetaminophen 151 16 g mol and acetic acid 60 052 g mol as shown in the reaction below What is the theoretical yield of acetaminophen CH3 suzu CH3 HO HC A 0 7627 g 1 610 g 3 126 9 1 920 g CH p aminophenol NH H C acetic anhydride HO HC NH CH HO acetaminophen acetic acid CH3

Physical Chemistry
General2 Metal bicarbonates on heating get decomposed to form some basic compounds and gas X Cold and concentrated solution of sodium chloride reacts with ammonia and gas X to produce an acidic salt Y and a basic salt Z Which of the following is not true about Z 0 8 1 It is sparingly soluble in water 2 It has two water molecules as water of crystallisation 3 It is used in soda acid fire extinguishers 4 It releases gas X on heating 4
Physical Chemistry
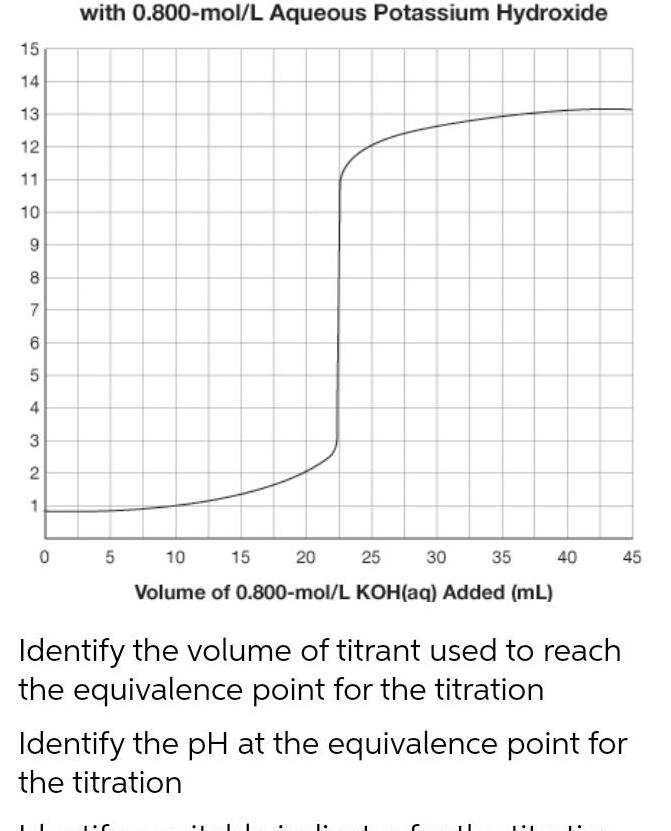
General15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 with 0 800 mol L Aqueous Potassium Hydroxide 5 10 15 20 30 35 40 25 Volume of 0 800 mol L KOH aq Added mL Identify the volume of titrant used to reach the equivalence point for the titration 45 Identify the pH at the equivalence point for the titration

Physical Chemistry
GeneralFor the reaction H g CO g CO g H O g if the initial concentration of H CO and moles litre of hydrogen is consumed at equilibrium the correct expression of Kp x is a 2 x b 1 x 1 x c 1 x d 2 x 2 1 x

Physical Chemistry
GeneralThere is a 190 kg sample in the natural state It dries in the oven and its weight is noted 186kg The absorption test is carried out and its SSD weight equal to 194kg is determined The specific weight of the material is equal to 2350 kg m and its unit weight is 1750 kg m Decide Moisture content absorption water total water free water absolute volume apparent volume void volume and of sample voids

Physical Chemistry
GeneralQuestion 2 19 marks Answer the questions below that relate to the structure and reactivity between the following species Br OH NH a CIRCLE the most nucleophilic site of all the sites in the neutral structures shown above

Physical Chemistry
General6 In case the membrane potential of mitochondrion is 150 mV pH inside a matrix is 7 0 while in intermembrane space 6 5 find how much protons mol can be transferred at standard biological conditions T 310 K pH 7 0 if 5 mol NADH is oxidized with the molecular oxygen

Physical Chemistry
GeneralWhen n hexene is heated with Mo203 a t 500 C and 20 atmospheres the follow ing changes takes place A Hybridization of carbon changes B No of C C bond increases C No of C H bond decreases D Percentage of C increases a A B only b C D only c A B C onl y d A B C D

Physical Chemistry
General1ST YEAR ISHIKA JAIN JANHAVI SINGH YASHSWANI MISHRA MANAN AGRAWAL KAUSHIKI GUPTA PRASHU SHUKLA VARTIKA CHAUHAN NIKARIKA CHAUHAN NISHTHA BHATNAGAR ISHA PARIHAR ISHITA SINGH RUDRANI DUTT MEHAK SHARMA SAKSHI SHUBHRA YADAV KRITIKA TIWARI SHYAMA PODDAR SWAPNIL RAI YASHIKA TOMAR PRANJAL PANDEY NANDINI GOEL AKSHITA SHARMA Official Literary Society CSE ESE IT CSE IT CSE CSE CSE CS CSE EE CSE CSE ECE CS CS EE CSE CS CSE CSE CS RECRUITMENTS 2022 MMDMETMDMTEMMEDME E EMME 2ND YEAR CSE ASTITV GUPTA KANCHI IT CSE GOPAL GUPTA ANADEE CSE IT KSHITIJ YADAV MANASWINI CSE PRATYUSH TRIPATHI IT SUYASH RASTOGI ECE HARSHIT SHUKLA CSE ABHISHEK TIWARI ECE


Physical Chemistry
GeneralIdentily comply bromine dissolved in carbon tetrachloride 1 It is a solid liquid homogeneous mixtu re 2 After separation bromine cannot be br oken down into simpler substances 3 Carbon tetrachloride has the propertie s of carbon and chlorine 4 Bromine and carbon tetrachloride can be separated by a separating funnel Can you please explain the answer to this q

Physical Chemistry
GeneralName the product of chemical reaction of but 1 3 diene with chlorine 1 2 dichlorobutane 1 3 dichlorobut 1 ene 1 4 dichlorobutane O 1 4 dichlorobut 2 ene 1 2 dichlorobut 2 ene

Physical Chemistry
GeneralConsider the following chemical equation 2NaOH H SO4 Na SO 2H O The informations conveyed by this equation are 1 NaOH reacts with H SO to produce Na SO and water 2 For every one molecule of H SO4 two molecules of NaOH are required III Acids and bases are non ionic in nature IV This is not a redox reaction The correct statements are a I and II b II and III

Physical Chemistry
GeneralDescribe the position of electrons around one O atom in the Lewis structure of O Select one O a 3 shared electron pairs Ob 3 shared electron pairs 1 lone pair O c 2 shared electron pairs 2 lone pairs O d 1 shared electron pair 3 lone pairs O e 2 shared electron pairs 1 lone pair 4

Physical Chemistry
Generalonsider the following statements 1 137 a Velocity of electron is th velocity of light b First line of Balmer series will be more intense compared to second line c Radius of third orbit of Li2 ion is 1 5 times more than radius of second orbit of He ion Correct among the following is are 1 a only 2 b c only 3 a c only 4 a b c are correct

Physical Chemistry
GeneralPrepare a control chart and evaluate whether or not the data meet each criterion for stability in control chart Sample Recovery Sample Recovery Sample Recovery 1 94 6 10 104 6 18 104 6 2 93 1 11 123 8 19 91 5 3 100 0 12 93 8 83 1 4 122 3 13 80 0 100 8 5 120 8 14 99 2 123 1 6 93 1 15 101 5 96 2 7 117 7 16 74 6 96 9 8 96 2 17 108 5 102 3 73 8 9 20 21 22 23 24 25

Physical Chemistry
GeneralA student at GCC made a saturated solution of Ca 103 2 by adding 256 gr of Ca 103 in 2 0 L of pure water Calculate the molar solubility of this salt at 298 K Ksp of Ca 103 2 2 0 x 10 6 O 3 9 x 10 3 O 7 9 x 10 3 O 4 4 x 10 3 O We can t tell since too much salt is in water 6 6x10 3

Physical Chemistry
GeneralSuppose you have 600 0 grams of room temperature water 20 0 degrees Celsius in a thermos You drop 90 0 grams of ice at 0 00 degrees Celsius into the thermos and shut the lid a What is the equilibrium temperature of the system Provide your final answer with three significant digits of precision here b How much ice is left in grams

Physical Chemistry
GeneralA 1 000 g sample of an alum is dissolved and sulfate is precipitated as BaSO The precipitate weighed 0 466 g after it was washed dried ware in the form K Al SO 24H O calculate the percentage if ALO in the sample Given Atomic mass of Ba 137 S 32 Al 27 alum A 3 4 B 13 2 C 1 7 D 6 8

Physical Chemistry
GeneralAn experiment calls for 185 mL of a 1 55 M solution of hydrochloric acid Stock solutions of concentrated hydrochloric acid are 12 4 M How many mL of stock solution would need to be diluted in order to make the solution required for the experiment O 14 8 mL O 23 1 mL O 2 31 x 102 mL O 9 59 x 10 3 mL O 1 48 mL

Physical Chemistry
GeneralCI None of these acid halide anhydride least reactive acid halide ester amide anhydride most reactive least reactive amide anhydride acid halide ester most reactive least reactive acid halide anhydride ester amide most reactive least reactive amide ester anhydride acid halide most reactive least reactive ester amide anhydride acid halide most reactive

Physical Chemistry
General35 Match the metal Column I with its reaction with oxygen Column II A B a Column I Potassium b x c i ii iii iv A iv B iii C ii D i A iv B ii C i D iii A iii B ii C i D iv A iv B ii C iii D i C D Silver Zinc Copper Column II Does not react event at high temperatures Gets coated with black coloured layer of oxi Does not burn at ordinary temperature Burns vigorously

Physical Chemistry
Generala For pure oxygen O2 gas at 0 C calculate the molecular density in the form of molecule cm3 in the following cases i and ii b In the following cases i and ii what is the average velocity of the N2 molecule 1 mole of oxygen atoms 16 g i at standard atmospheric pressure 1013 mbar normal pressure is accepted ii at 10 10 mbar pressure UHV and 1000 km above the ground surface Assume O2 gas behaves as an ideal gas

Physical Chemistry
GeneralAn iron tank contains helium at a pressure Example 5 6 of 2 5 atmosphere at 25 C The tank can withstand a maximum pressure of 10 atmosphere The building in which tank has been placed catches fire Predict whether the tank will blow up first or melt The melting point of iron 1535 C
Physical Chemistry
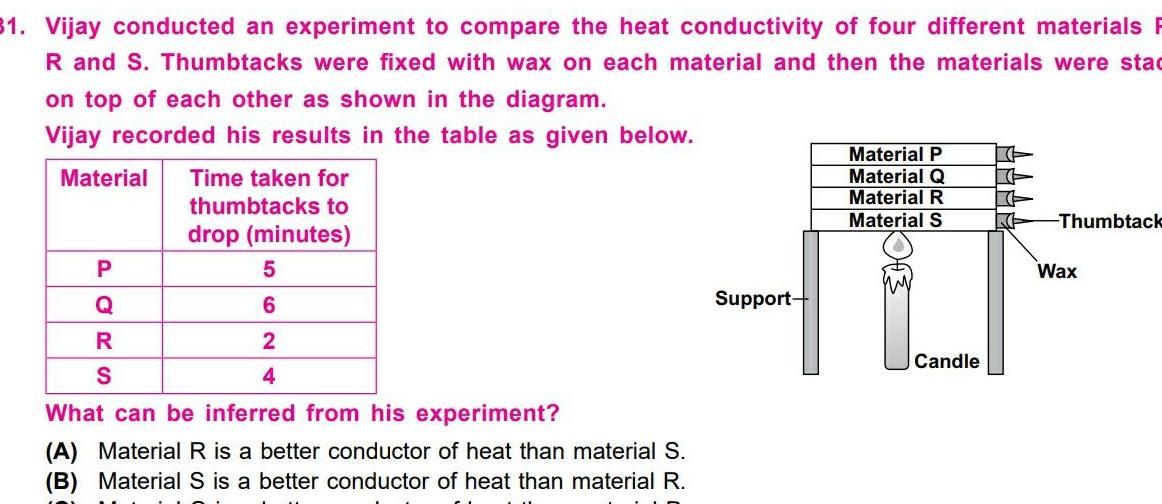
General31 Vijay conducted an experiment to compare the heat conductivity of four different materials R and S Thumbtacks were fixed with wax on each material and then the materials were stad on top of each other as shown in the diagram Vijay recorded his results in the table as given below Material P Q R S Time taken for thumbtacks to drop minutes 5 6 2 4 What can be inferred from his experiment A Material R is a better conductor of heat than material S B Material S is a better conductor of heat than material R Support Material P Material Q Material R Material S Candle K Thumbtack Wax

Physical Chemistry
General4 Use the Universal Soil Loss Equation USLE and the tables and graphs of values for USLE variables in Chapter 17 of Brady Weil to a determine if a 150 meter long tilled row crops field of Mexico silt loam located in McCredie Missouri on a 4 slope is classified as highly erodible land HEL The definition of highly erodible land is given in the footnote at the bottom of page 874 15th ed Assume the T value for this soil is 11 Mg ha yr

Physical Chemistry
GeneralCalculate the relative population of the J 1 rotational level of the ground vibrational state of HCl at 300 K Use the values Planck s constant h 6 626x10 Js velocity of light c 2 998 10 ms constant for the diatomic molecule B 10 4398 cm and k 1 3806 10 JK A 2 71 B 0 C 1 2 71 D 1 Answer OA OB OC OD questio Submit

Physical Chemistry
GeneralA2 3 7 5w Two substance A B are allowed to react completely to from A B A B mixture of leaving 1 4 none of the reactants Using this information calculate the composition of final mixture when mentioned amount of A B are taken 1 6 2 4 1 3 2 32 5 If moles of A 2 moles of B is taken 4 A A B 0 2 mole A B 0 3 mole A B 0 5 mole A B 0 5 mole If 7 moles of A 6 moles of B is taken A A B 1 mole A B 2 C A B 2 mole A B 4 If 4 moles of A 6 moles of B is taken A A B 2 mole A B 3 C A3B 4 mole A B 1 1 5 32 32 5x B A B4 0 8 mole A B 0 7 mole D A3B4 0 4 mole A B 0 6 mole z B A B 2 mole A B 1 D A B 4 mole A B4 1 x x S 2 B A B 2 mole A B 1 D A3B4 3 mole A B 2 7 2 X 1 07 21x4

Physical Chemistry
GeneralWhat is the change in internal energy when a gas contracts from 377 mL to 177 mL under a constant pressure of 1520 tort while at the same time being cooled by removing 124 J heat 40 52 J O 83 48J O 248J None of these

Physical Chemistry
GeneralA solid substance A decomposes into two gaseous product B and C as A 2B g C g If at equilibrium some C at 1 atm is added in constant volume condition 10 of Bg solidified before the equilibrium was re established What is the total pressure at final equilibrium

Physical Chemistry
Generalb Given that the value of Kc is 5 2 x 10 at 8592 K for which of the following concentrations of molecular gas would the small x approximation be valid x is 5 of the initial concentration or less 1 mark Select all that apply noting there is a penalty for incorrectly selected responces 0 01 M 0 03 M 0 1 M 0 3 M 1 M U 3 M 10 M None of these concentrations are suitable c At this temperature what is the concentration of oxygen atoms at equilibrium in a sealed chamber initially containing only

Physical Chemistry
GeneralHERE IT IS GIVEN THAT THE REACTION MU ST BE AT CONSTANT PRESSURE SO THE C ONTAINER MUST BE OPEN BUT IT ISNT O PEN HERE asurements at constant pressure For the measurement of heat transferred at constant pressure the calorimeter used should constitute an open system So any vessel kept open can be used along with a thermometer for the measurement of the temperature change and a stirrer for distributing the heat released throughout the solution The apparatus is shown in Fig 3

Physical Chemistry
General12 a Draw the kinetic and thermodynamic enolates of the ketone below 4 points A Kinetic enolate B Thermodynamic enolate C b On the reaction coordinate diagram identify which enolate fits in each empty box E IN

Physical Chemistry
GeneralThe magnetite is a mixed oxide of inon It consists of Feo and Fe 03 Assume that given sample was lying in contact with oxidising agent it contains more Fie 03 than Feo Given sample of magnetite on reaction with carbon monoxide forom iron metal and co If 39 2g of given sample of magnetite on reaction with sufficient carbon monoxide produces 15 68 L of CO gas at S T P Then 3 9 2g of given sample That is given in paragraph is completely oxidised into Fe by KMNO4 and K G 07 in different experiment x ml of 0 1 M KMNO4 Cin acidic medium is required for Complete oxidation of given sample while y ml of 0 1 M K C 07 is required for Complete oxidation Cin acidic medium select the incorrect statement a x 200 b y 166 67 771

Physical Chemistry
General2 Example 7 9 1 0 mol of H 2 0 mol of 1 and 3 0 mol of HI are injected in a 1 litre flask What will be the concentration of H I2 and HI at equilibrium at 490 C The equilibrium constant for the reaction at 490 C is 45 9

Physical Chemistry
GeneralIn a serine protease cysteine can be substituted for serine and still retain nearly full catalytic activity What property of cysteine permits this A B C D When deprotonated the thiol group is a good nucleophile like the hydroxyl group The sulfur of cysteine is nearly the same size as the oxygen in serine Preferential binding of the transition state is the primary driver of the reaction Both A and C