Atomic Structure Questions and Answers

Physical Chemistry
Atomic Structure23 Which combinations of quantum numbers n I m and s for the electron in an atom does not provide a permissible solutions of the wave equation 1 3 2 22 3 3 21 2 3 3 1 1 2 4 3 11 2

Physical Chemistry
Atomic StructureBohr s theory accounts for the stability and line spectrum of Li ion Bohr s theory was unable to explain the splitting of spectral lines in the presence of a magnetic field n the light of the above statements choose the most appropriate answer from the options iven below Statement I Statement II Statement I is false but statement II is true Both statement I and statement II are false Both statement I and statement II are true Statement I is true but statement II is false Question Type MCQ Question ID 86435156 Option 1 ID 86435116

Physical Chemistry
Atomic Structure21 The correct order of energy difference between adjacent energy levels in H atom 1 E E E E E E 2 E E E E E E 3 E E E E E E 4 E E E E E E

Physical Chemistry
Atomic Structure29 Which is the correct graphical representation based on photoelectric effect L K E K E Intensity of light 1 1 11 3 III IV II IV KE No of photons Intensity of light 2 11 1 4 11 IV

Physical Chemistry
Atomic StructureWhich of the following statements is not correct for ar electron that has the quantum numbers n 4 and m 2 a The electron may have the quantum number s MNR 1993 1 2 b The electron may have the quantum number 1 2 c The electron may have the quantum number 1 3 d The electron may have the quantum number 1 0 1 2 3

Physical Chemistry
Atomic Structuretransition is inversely related to difference in the energy levels involved in the transition A Zn II salts are diamagnetic R Zn ion has one unpaired electron

Physical Chemistry
Atomic StructureA Electronic energy for hydrogen atom of different orbitals follow the sequence 1s 2s 2p 3s 3p 3d R Electronic energy for hydrogen atom depends only on n and is independent of r m values

Physical Chemistry
Atomic Structure6 The Bohr orbit radius for the hydrogen atom n is approximately 0 530 A The radius for the excited state n 2 orbitis in A 1 4 77 2 1 06 3 0 13 4 2 12

Physical Chemistry
Atomic Structure1 st 43 The frequency of radiation emitted when the 4 to n 1 in a hydrogen ionization energy of and h 6 625 x 10 4 Js electron falls from n atom will be Given H 2 18 10 8 J atom 1 1 54 x 1015 1 2 1 03 1015 S 1 3 3 08 x 1015 s 1

Physical Chemistry
Atomic StructureQ23 Which L C A O of two O atoms produce given shape molecular orbitals If internuclear axis is ID Q 529170 Options are A 2s and 2s Wave functions are added B 2px and 2px C 2py and 2py D 2p and 2pz Wave functions are added Wave functions are added Wave functions are substracted from the other

Physical Chemistry
Atomic Structure16 Number of spectral lines falling in Balmer series when electrons are de excited from th shell will be given as 1 n 2 in UV 2 n 2 in visible region 3 n 3 in near IR 4 n 3 in far IR 17 The ratio of the energy required to remove an electron

Physical Chemistry
Atomic StructureQ15 For the electrons of oxygen atom which of the following statements is correct a Zeff for an electron in a 2s orbital is the same as Zeff for an electron in a 2p b An electron in the 2s orbital has the same energy as an electron in the 2p c Zeff for an electron in Is orbital is the same as Zeff for an electron in a 2s orbital

Physical Chemistry
Atomic StructureR Orbital angular momentum depends on orientation of orbitals A Energy of electron is taken negative R Energy of electron at infinity is zero

Physical Chemistry
Atomic Structurena Bohr s model of an atom when an umps from n 1 to n 3 how much ene me emitted or absorbed 1 2 389 10 12 ergs 2 0 239 x 10 10 ergs 3 2 15 x 10 11 ergs 4 0 1936 10 10 ergs

Physical Chemistry
Atomic Structure30 The energy of second Bohr orbit of the hydrogen a is 328 kJ mol hence the energy of fourth E orbit would be AIPMT Prelims 20 1 41 kJ mol 2 1312 kJ mol

Physical Chemistry
Atomic Structure3 If each orbital can hold a maximum of 3 electrons the number of elements in 4th period of periodic table long form is a 48 2 27 b 54

Physical Chemistry
Atomic Structure1 19 Ferric oxide crystallises in a hexagonal close packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions Derive the formula of the ferric oxide 1 20 Classify each of the following as being either comiconductor ile9 p type or a n type

Physical Chemistry
Atomic Structure5 The energy of electron in first energy level is 21 79x 10 12 erg per atom The energy of electron in second energy level is a 54 47 x 10 12 1 erg atom b 5 447x 10 2 1 erg atom c 0 5447 10 2 erg atom 1 d 0 05447x 10 12 erg atom 1

Physical Chemistry
Atomic StructureThe number of waves made by a Bohr electron in H atom for one complete revolution in its 3rd orbit are A 1 B 2 C 3 D 4

Physical Chemistry
Atomic StructureIf in a photoelectric cell the wavelength of incident light is changed from 4000 A to 3000 A then change in stopping potential will be 1 0 66 V 2 1 03 V 3 0 33 V 4 0 49 V a Pusa Road New Delhi 110005 Ph 011 47623456

Physical Chemistry
Atomic StructureWhich of the following statements is correct 1 The electronic configuration of Cr is Ar 3d 4s Atomic No of Cr 24 2 The magnetic quantum number may have a negative value 3 In silver atom 23 electrons have a spin of one type and 24 of the opposite type Atomi of Ag 47 4 All of the above

Physical Chemistry
Atomic Structure8 An organic compound containing C H and N gave the following analysis C 40 H 13 33 N 46 67 Its empirical formula would be 1 CH N 3 C H N 2 CH N 4 C H N

Physical Chemistry
Atomic StructureThe radial probability function which provides us the probability of finding an electron in a spherical shell of thickness dr at a distance of r from the nucleus for the hydrogen atom is plotted here for an orbital having a value of O for I The orbital is 10 40 30 20 10 2S 2Pz 3d O 1 0 1 point 200 400 600 800 10001200 1400

Physical Chemistry
Atomic StructureChoose the correct statement among the following Radial distribution function y 4r dr give probability at a particular distance along one chosen direction p r give probability density at a particular distance over a spherical surface 1 11 III IV A II IV For s orbitals P r Y 0 T T x y z is independent of 9 and 2p orbital with quantum numbers n 2 2 1 m 0 also shows angular dependence B II III IV C I III IV D III N

Physical Chemistry
Atomic Structure3 A mixture of methane and ethene in a molar ratio ofx y has an average molecular mass of 20u The mean molar mass when they are mixed in the molar ratio y x will be 2 25 u 3 24 u 1 20 u 4 15 u

Physical Chemistry
Atomic StructureMr Santa has to decode a number ABCDEF where each alphabet is represented by a single digit Suppose an orbital whose radial wave function is represented as Yk ek 5kr 2 6k From the following information given about each alphabet then write down the answers in the form of ABCDEF for above orbital Info A Value of n where n is principal quantum number Info B Number of angular nodes Info C Azimuthal quantum number of subshell to orbital belongs Info D Number of subshells having energy between n 5 s to n 5 p where n is principal quantum number Info E Orbital angular momentum of given orbital Info F Radial distance of the spherical node which is farthest from the nucleus Assuming k3 1
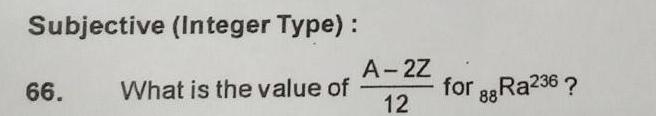
Physical Chemistry
Atomic StructureSubjective Integer Type 66 What is the value of A 2Z 12 for Ra236 88

Physical Chemistry
Atomic StructureIn face centred cubic lattice tetrahedral voids are present at O each face diagonal O each cube diagonal O body centre Orach nin

Physical Chemistry
Atomic StructureOn absorbing light of wavelength 3800 bromine molecule undergoes dissociation and form atoms The kinetic energy of one bromine atom assuming that one quantum of radiation is absorbed by each molecule would be Bond energy of Br 190 kJ mol 1 1 04 x 10 19 J 2 2 08 x 10 19 J 4 6 25 x 104 J 3 1 25 x 10 5 J

Physical Chemistry
Atomic StructureIn compounds of type ECI where E is B P As or Bi The angles CI E CI for different E are in order 2 B P As Bi 3 B P As Bi 4 B P As Bi 1 B P As Bi For the reaction Cl 211 2C the initial concentration of I was 0 20 mol I and the concentration after 20 min was 0 18 mol Z Then the rate of formation of 1 in mol L min

Physical Chemistry
Atomic Structure3 865 58 Cal 865 58 Cal 4 0 865 58 Cal The angular momentum of an electron in a Bohr s orbit of He is 3 1652x10 kg m sec What is the wave number is terms of Rydberg constant R of the spectral line emitted when an electron falls from this level to the first excited state Use h 6 626x10 Js 1 3R 2 SR 3R 3 31 8R 9

Physical Chemistry
Atomic StructureThe de Broglie wavelength asociated with particle of mass 10 kg moving with a velocity of 10ms is AIIMS b 6 63x10 16 d 6 63x10 29 a 6 63x10 7 c 6 63 10 21

Physical Chemistry
Atomic StructureFor sodium atom number of electrons with m 0 will be 1 2 2 7 3 9 4 8

Physical Chemistry
Atomic StructureEqual moles of hydrogen and oxygen gases are placed in a container with a pin hole through which both can escape What fraction of the oxygen escapes in the time required for one half of the hydrogen to escape NEET 2016 11 12 2 1 2 NI 4 7 100 8 1 3 4 3

Physical Chemistry
Atomic StructureFor radial function angular function correct option is are A R r determine size of an orbital B angular function determine energy C R r contains n m only D q q contains m

Physical Chemistry
Atomic Structure2 3 2 Suppose the elements X and Y combine to form two compounds XY and X Y When 0 1 mole of XY2 weighs 10 g and 0 05 mole of X Y weighs 9 g the atomic weights of X and Y are a 40 30 c 20 30 b 60 40 d 30 20

Physical Chemistry
Atomic StructureATOMIC STRUCTURE In hydrogen atom the kinetic energy of electron is 3 4 eV The distance of that electron from the nucleus 2 0 529A 4 21 16A 1 2 116A 3 1 587A

Physical Chemistry
Atomic StructureThe ground state energy in J of hydrogen atom is X The minimum energy in J required to promote an electron from n 1 to n 2 in Het is 4X 1 3X 2 3 3 3X 4 3

Physical Chemistry
Atomic Structureprobability density plots of ls and 2s orbitals are given in figure O 1s O 2s The density of dots in a region represents the probability density of finding electrons in the region On the basis of above diagram which of the following statements is incorrect a 1s and 2s orbitals are spherical in shape b The probability of finding the electron is maximum near the nucleus c The probability of finding the electron at a given distance is equal in all directions d The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus increases

Physical Chemistry
Atomic Structure2 In ion dipole interaction potential energy depends on the distance between interacting particles which of the following relation is correct a U x 2 2 c U x 6 r b Uc d Uc 4 Y r

Physical Chemistry
Atomic StructureThe valence shell electronic configuration transition elements is 151 1 ns 2 ns np5 3 ns0 2 n 1 d1 10 4 ns n 1 10

Physical Chemistry
Atomic Structurea The schrodinger wave equation for hydrogen atom is 1 2 To r 2ag ao Wherea is Bohr s radius Let the radial node in 2s be at r Then find r in terms of a 4 2s 1 1 2 2 ao 2

Physical Chemistry
Atomic StructureThe value of spin only magnetic moment for one of the following configuration is 2 84 B M The correct one is a d in strong field ligand b d in weak field ligand c d in weak as well as in strong field ligand d d in strong field ligand
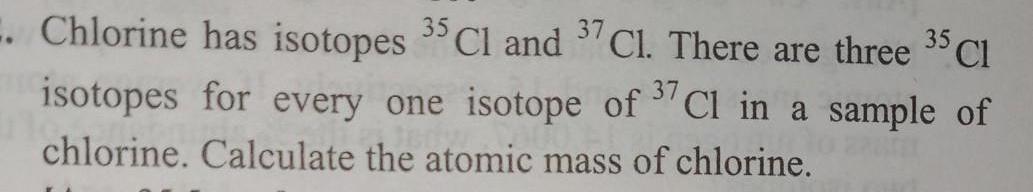
Physical Chemistry
Atomic StructureChlorine has isotopes 35 Cl and 37 Cl There are three 35 Cl isotopes for every one isotope of Cl in a sample of chlorine Calculate the atomic mass of chlorine 37

Physical Chemistry
Atomic StructureWhat is the maximum number of orbitals that can be identified with the following quantum numbers n 3 1 1 m 0 AIPMT 2014 1 01 Bel 2 2 4 4 1 1 3p orbital 3 3 11 41 LL L m

Physical Chemistry
Atomic StructureO Calculate wavelength of an electron moving with a velocity of 2 05 107 ms Mass of electron 9 1 10 31 kg

Physical Chemistry
Atomic Structure1 22 10 eV Calculate the energy of photon in kcal mol while wave number of photon is 1 cm Wave Moun number 1cm 1 10 m 10 m 1

Physical Chemistry
Atomic StructureJEE Advance 2019 Answer the following by appropriately matching the lists based on the information given in the paragraph Consider the Bohr s model of a one electron atom where the electron moves around the nucleus In the following List I contains some quantities for the nth orbit of the atom and List II contains options showing how they depend on n List I Radius of the nth orbit Angular momentum of the electron in the nth orbit Kinetic energy of the electron in the nth orbit Potential energy of the electron in the nth orbit 1 III IV List II P Q xn m n x n n xn 2 Which of the following options has the correct combination considering List I and List II A IV Q B III P C IV U D III S S T Which of the following options has the correct combination considering List I and List II A 1 T B II Q D II R C 1 P

Physical Chemistry
Atomic Structure11 Ionization energy for hydrogen atom in ergs Joules and eV respectively is Inqining b 13 6 x 218 10 20 21 8 10 3 RECO d 21 8 x 10 3 13 6 21 8 10 20 218 10 20 13 6 a 21 8 x 10 2 218 10 c 21 8 10 20 13 6 21 8 x 10 13

Physical Chemistry
Atomic StructureElectronegativity of the following elements increases in the order 1 C N Si P 3 Si P C N 2 N Si C P 4 P Si N C