Atomic Structure Questions and Answers

Physical Chemistry
Atomic StructureWhich graph is true for 4p orbital of H like atom RPF Radial probability function H 4 RPF A RPF B RPF C RPF mn D RPF Your Answer D orrect AnswOK

Physical Chemistry
Atomic StructureConsider a two level system of particles that is each particle can either be in the ground or the exited state The energy separation is A 2 kB 500K At what temperature 7 will the population of the exited state be that of the ground state At what temperature will the population of the two states equal

Physical Chemistry
Atomic Structure1 Number of significant figures in 10 3406g is 2 2 4 6 15 HO 3 3 ACES 74 5g of a metallic chloride contains 35 5g of chlorine The exact atomic weight of the metal is x and its specific heat is 0 0406 Cal g C The value of x is 1 150 3 156 9 Nitrogen forms two c law can be proved b 1 Constant Comm 2 Multiple Prop 3 Reciprocal 4 Conservati 10 x mole ator atoms The 2 157 6 4 155 02 mole of a diatomic 1 0 05 10

Physical Chemistry
Atomic StructureWhich of the following statement is are CORRECT A cation is smaller than an anion if they are isoelectronic Out of second period anion fluoride ion is large in size Both Mn and Co are paramagnetic Generally metallic radius of an element X is higher than its covalent radius

Physical Chemistry
Atomic StructureWhich of the following statement is INCORRECT Li is harder than the other alkali metals In Solvay process NH3 is recovered when the solution containing NH CI is treated with H O Na CO is pearl ash Beryllium and aluminium ions do not have strong tendency to form complexes like ReF
Physical Chemistry
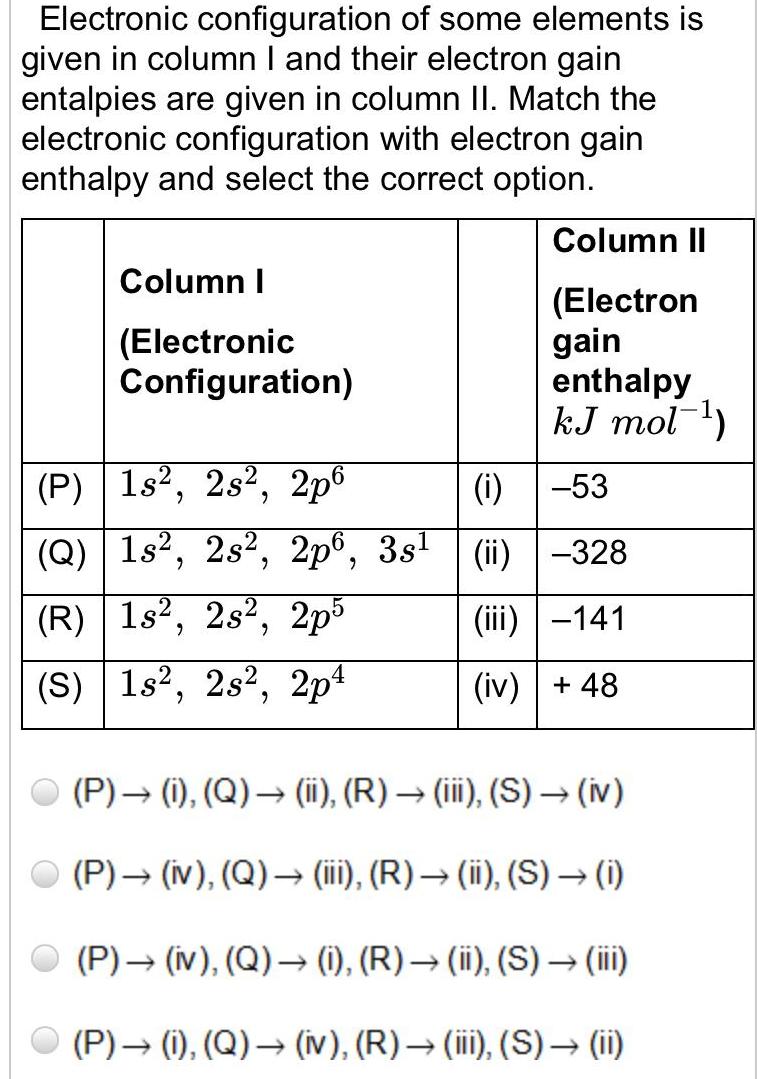
Atomic StructureElectronic configuration of some elements is given in column I and their electron gain entalpies are given in column II Match the electronic configuration with electron gain enthalpy and select the correct option Column I Electronic Configuration P 1s 2s 2p6 Q 1s 2s 2p6 3s R 1s2 2s2 2p5 S 1s2 2s2 2p4 Column II Electron gain enthalpy kJ mol i 53 ii 328 iii 141 iv 48 P i Q ii R iii S iv P iv Q iii R ii S i P iv Q i R ii S iii P i Q iv R iii S ii

Physical Chemistry
Atomic Structurection is C 1 42 An element A in a compound ABD has oxidation number A It is oxidised by Cr O2 in acidic medium In the were used for number experiment 1 68 x 10 3 mole of K Cr O7 3 26 10 3 mole of ABD The new oxidation A after oxidation is a 3 c n 3 b 3 n d n

Physical Chemistry
Atomic StructureWhich of the following is wrong a Cathode rays have constant e m ratio b e m ratio of anode rays is not constant c e m ratio of protons is not constant d e m ratio of particles is constant

Physical Chemistry
Atomic Structurepossible values of n l and m for this electron Question Type Single Correct Type 1 n 3 l 0 m 0 2 n 3 l 1 m 1 0 1 3 n 3 l 2 m 2 1 0 1 2 4 n 3 l 3 m 3 2 1 0 1 2 3 ne

Physical Chemistry
Atomic StructureWhich of the following information is not correct about principal quantum number n 1 As value of n increases the energy of the orbit increases 2 As value of n increses the distance from the nucleus increases 3 As value of n increases the velocity of electron increases 4 The maximum number of electrons in a particular shell is equal to 2n

Physical Chemistry
Atomic Structure68 The wavenumber of a radiation whose frequency is 7 5 10 s 1 is 1 1 25 m 1 2 0 25 m 1 3 2 5 m 1 4 25 m 1 69 The species which has the maximum charge to mass ratio is 1 Electron 3 Neutron 2 Proton 4 a particle

Physical Chemistry
Atomic Structure2 Energy and size of the shell is determined by 1 Principal Quantum Number 2 Azimuthal Quantum Number 3 Magnetic Quantum Number 4 Spin Quantum Number 3 The ratio of the wavelength of the first line of Balmer series to that of the first line of Paschen series of hydrogen atom is 1 7 20 2 20 7 3 25 9 4 8 27

Physical Chemistry
Atomic StructureWhich of the following statements is not correct regarding to galvanic cell Question Type Single Correct Type 1 Cell reaction is spontaneous from left to right if Ecell 0 2 Cell reaction occurs from right to left if Ecell 0 If the system is at equilibrium no net reaction 3 occurs 4 Ecell is temperature independent

Physical Chemistry
Atomic StructureThe orbital angular momentum of an electron present in 3p orbital is 1 2h 2 Zero 3 6 h 4 2 2h Maximum number of orbital s in an atom that have quantum number n 4 1 1 m 1 is 1 4 3 2 2 3 4 1

Physical Chemistry
Atomic StructureTest 01 Code A 84 In which of the following orbital diagrams Pauli s exclusion principle Hund s rule of maximum multiplicity and Aufbau principle all are violated 3s 1 1 2 1 3 1 4 1 85 S is isoelectronic with 1 Ca 3 Na 3p 11 1 1 1 111 1 1 1 2 K 4 Mg2

Physical Chemistry
Atomic StructureIn the Bohr s model for unielectronic species following symbols are used Radius of nth orbit with atomic number z Potential energy of electron in nth orbit with atomic number z Kinetic energy of electron in nth orbit with atomic number z Velocity of electron in nth orbit with atomic number z Time period of revolution of electron in nth orbit with atomic number z Calculate z in all in cases 8 1 Pn z Unz n z V n z T n z K1 2 1 U 2 K z iii V V 1 9 1 V3 ii 2 12 1 1 8 iv T 2 T z 9 32 T2

Physical Chemistry
Atomic Structure54 55 An organic compound A contains 20 C 46 66 N and 6 66 H It gave NH gas on heating with NaOH The organic compound A could be 1 CH CONH 2 C H CONH 3 NH CONH 4 CH NHCONH If the temperature of an ideal gas in a sealed rigid container is increased to 1 5 times the initial value

Physical Chemistry
Atomic Structured 4 0 51 The most probable velocity of a gas molecule at 298 Ki 300 m sec Its RMS velocity in m s is a 420 b 245 c 402 d 367 52 The ratio of
Physical Chemistry
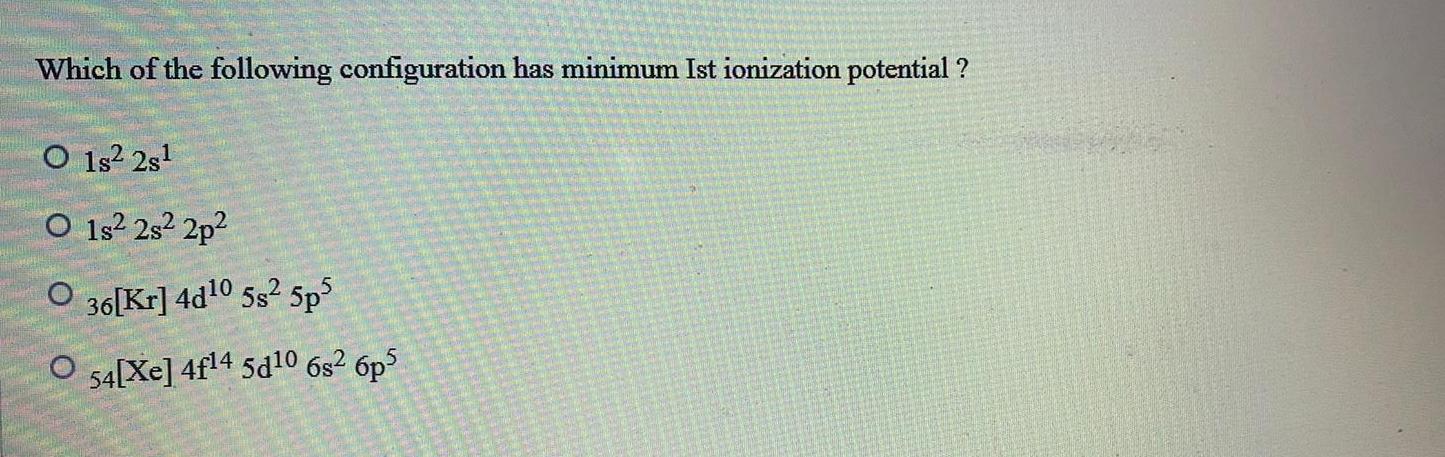
Atomic StructureWhich of the following configuration has minimum Ist ionization potential O1s 2s O1s 2s 2p O 36 Kr 4d 0 5s 5p5 O 54 Xe 4f14 5d10 6s 6p5

Physical Chemistry
Atomic Structure40 The ratio of de broglie wavelength of a deuterium atom to that of a particle when the velocity of former is five times greater than that of latter is a 4 b 10 2 c 2 d 0 4

Physical Chemistry
Atomic Structure35 Five ionization energy values in kJ mol are listed below 1010 E 1290 E 376 E 870 E 830 E 1010 E These are a Successive ionization energies for the element with atomic number 5 b The first I E of successive elements in group 15 16 17 18 and 1 respectively c The first I E of elements with atomic number 1 to 5 d Successive L E for transition elements with four electrons in d subshell

Physical Chemistry
Atomic Structure14 If you are given Avogadro s number of atoms of a gas X If half of the atoms are converted into X energy AH The IE of X is by 2AH NA AH 2NA a C 5 The ene b 2NA AH d NA AH

Physical Chemistry
Atomic Structure25 An electron in an atom undergoes transition in such a way that its kinetic energy changes from x to the change in potential energy will be 4 3 a x 2 3 C X 4 b 3 X 8 3 d x 4

Physical Chemistry
Atomic StructureThe nucleus of an atom is located at x y z 0 If the probability of finding an s orbital electron in a tiny volume around x P y z 0 is 7 10 4 The probability of finding of electron in the same sized volume around z P x y 0 14 x 10 4 7 x 10 14 7 x 10 4

Physical Chemistry
Atomic Structure34 The radius of the first orbit of hydrogen atom is 0 52 x 10 8 cm The radius of the first orbit of Het ion is a 0 26 10 8 cm c 1 04 x 10 8 cm b 0 52 x 10 8 cm d 2 08 x 10 8 cm 35 The ratio of ionigati

Physical Chemistry
Atomic Structurea 0 002 b 0 1 c 0 5 21 The ionisation energy of He is 19 6x 10 energy of the first stationary of Li2 is a 2 2 x 10 15 J atom b 8 82 x 10 17 J atom c 4 41 10 16 J atom 1 d 4 41 x 10 17 J atom d 0 7 J atom The urface

Physical Chemistry
Atomic Structure39 The uncertainty in the velocity of particle of mass 6 626x10 28 Kg is 10 m sec What is the uncertainty in its position in nm a 1 2 b 2 5 c 4 TC d YAT 4

Physical Chemistry
Atomic StructureThe ionization energy of He atom in the ground state may be a 13 6 eV b 54 4 eV c 108 8 eV The binding energy for the third electron in the ground d 27 0

Physical Chemistry
Atomic Structurec AR AR 1 d 2 1 96 Ionization potential of hydrogen atom is 13 6 eV Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy 12 1 eV According to Bohr s theory the spectral lines emitted by hydrogen will be a one c three b two d four last line is tot a c 100 The a b

Physical Chemistry
Atomic Structure4 0 g of caustic soda NaOH mol mass 40 contains same number of sodium ions as are present in a 10 6 g of Na CO mol mass 106 b 58 5 g of NaCl Formula mass 58 5 c 100 ml of 0 5 M Na SO4 Formula mass 142 d 1mol of NaNO mol mass 85

Physical Chemistry
Atomic Structure10 Two elements X and Y combine in gaseous state to form XY in the ratio 1 35 5 by mass The mass of Y that will be required to react with 2 g of X is b 3 55 g a 7 1 g c 71 g d 35 5 g

Physical Chemistry
Atomic StructureWhat minimum number of atoms ions should be present in a sample of H like species so that a maximum of 6 spectral lines can be produced of electronic transition from fifth excited state upto n 2 2

Physical Chemistry
Atomic Structures BOOKLET 1 In a mixture of sample of H atoms and He ions electrons in all the H atoms and He ions are present in n 4th state Then find maximum number of different spectral lines obtained when all the electrons make transition from n 4 upto ground state 4 1 32423

Physical Chemistry
Atomic StructureIn the Rutherford Gold foil experiment he bombarded alpha par ticle i e He nuclei He2 ion which contained 2protons and 2neutrons on a gold foil through which he acknowledged that most of the part of atom is empty and the mass is situated at nucleus of atom But when the He2 nuclei was bombarded it passed through the gold foil atoms which also contained elec trons so why the electrons didn t attracted the protons of the He2 ion instead it got scattered by different angles Because the protons of He2 nuclei and electrons of gold foil are of opposite charges and opposite charges attract each other

Physical Chemistry
Atomic Structuresion and Absorption Spectra The spectrum of radiation emitted by a substance that has absorbed energy is called an emission spectrum Atoms molecules or ions that have absorbed radiation are said to be excited To produce an emission spectrum energy is supplied to a sample by heating it or irradiating it and the wavelength or frequency of the radiation emitted as the sample gives up the absorbed energy is recorded An absorption spectrum is like the photographic negative of an emission spectrum A continuum of radiation is passed through a sample which absorbs radiation of certain wavelengths The missing wavelength which corresponds to the radiation absorbed by the matter leave dark spaces in the l

Physical Chemistry
Atomic Structure38 If the ionization energy of He is 19 6 10 18 J per atom then the energy of Be3 ion in x 34 second stationary state is a 4 9 x 10 18 J The energy of the second Rohr orbi in the br b 44 1x 10 18 J c 11 025 x 10 18 J d None of these

Physical Chemistry
Atomic StructureWhat minimum tube voltage is required to excite the KB and LB series of lines for rubidium Rb A 15 0 kV for KB 1 75 kV for LB B 1 75 kV for KB 15 0 kV for LB C 15 0 kV for KB 15 0 kV for LB D 1 75 kV for KB 1 75 kV for LB

Physical Chemistry
Atomic StructureTwo solids dissociate as follows A s B g C g Kp x atm D s C g E g K yatm P2 The total pressure when both the solids dissociate simultaneously is a x y atm c x y atm 2019 Main 12 Jan I b x y atm d 2 x y atm

Physical Chemistry
Atomic StructureFor 3s orbital of hydrogen atom the normalised wave function is given by 3 2 1 1 18r 2r 813 80 10 The above mentioned orbital 3s has two nodes at 2ao and xao Find the value of x 4 Use 3 3 5 W3s 27 C

Physical Chemistry
Atomic StructureMatch the parameters of column l with column Il as they are directly proportional Column l A f B T C of 15 E P Q R Column II n Z 1 2 n D S Z Where Frequency f Time period T Energy of nth orbit En radius of nth orbit r Atomic number Z Orbit number n
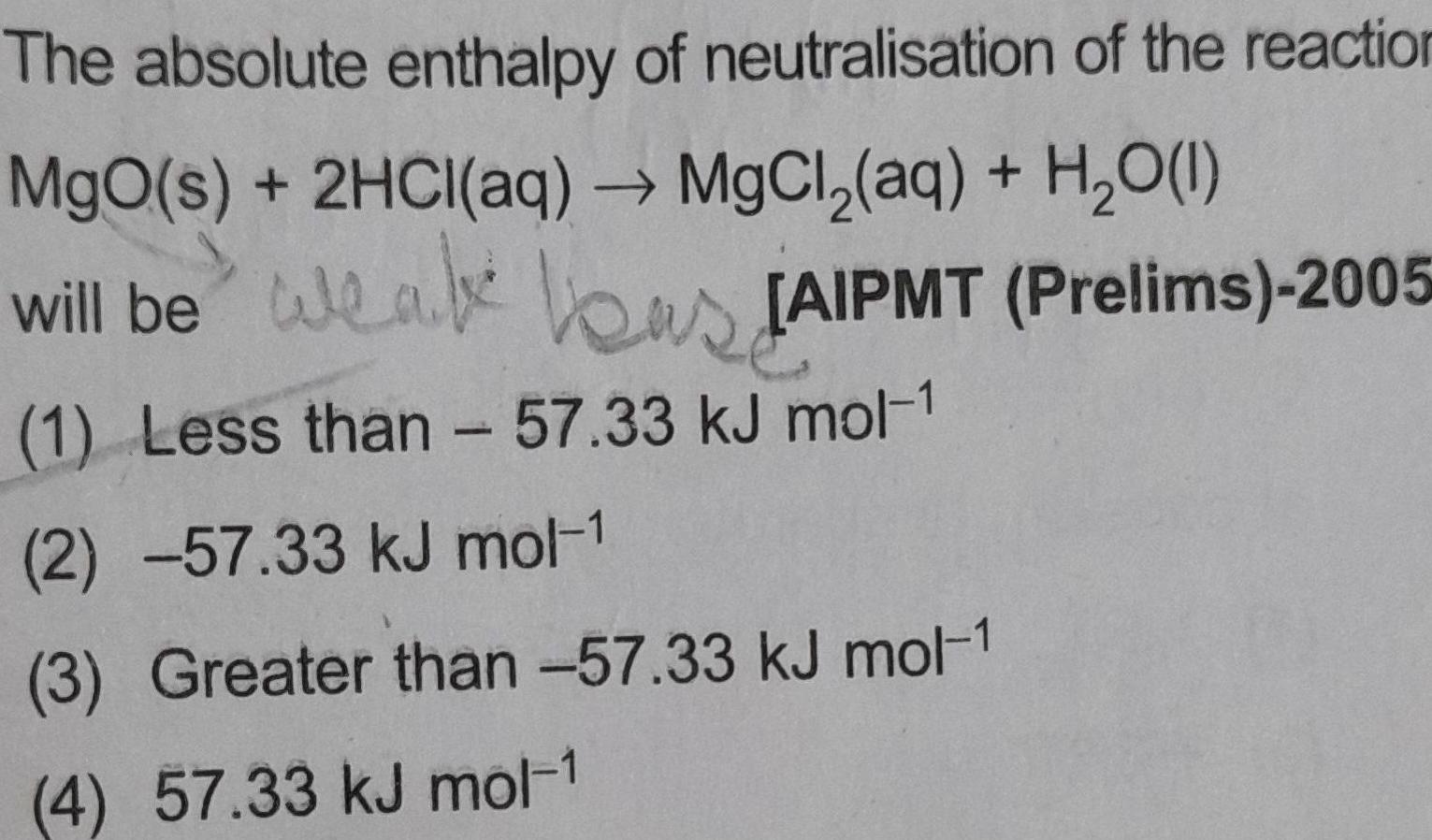
Physical Chemistry
Atomic StructureThe absolute enthalpy of neutralisation of the reaction MgO s 2HCl aq MgCl aq H O 1 will be weak as JAIPMT Prelims 2005 1 Less than 57 33 kJ mol 1 2 57 33 kJ mol 1 3 Greater than 57 33 kJ mol 1 4 57 33 kJ mol 1

Physical Chemistry
Atomic Structure1 30 The measurement of the electron position is associated with an uncertainty in momentum which is equal to 1 x 10 18 g cm s 1 The uncertainty in electron velocity is Mass of an electron is 9 10 28 g AIPMT Prelims 2008 1 1 x 1011 cm s 2 1 x 10 cm s 3 1 x 106 cm s 4 1 x 105 cm s 1 Consider the following cots of quantu The is orb 1 2 3 4 Questi 35 U 10

Physical Chemistry
Atomic StructureAssuming Heisenberg Uncertainity Principle to be true what could be the minimum uncertainty in de brogli wavelength of a moving electron accelerated by Potential Difference of 6 V whose uncertainty in position 7 22 A 6 25 A is n m B 6 A C 0 625 A D 0 3125 A

Physical Chemistry
Atomic Structure5 E 313 6 n2 kcal mole If the value of E 34 84 kcal mole to which value does n correspond 1 4 3 2 2 3 4 1

Physical Chemistry
Atomic Structure3 The ionization energy of the electron in the lowest orbit of hydrogen atom is 13 6 eV The energies required in eV to remove electron from three lowest orbits of hydrogen atom are 1 13 6 6 8 8 4 2 13 6 10 2 3 4 4 13 6 3 4 1 51 3 13 6 27 2 40 8

Physical Chemistry
Atomic StructureAccording to Bohr s theory En Total energy V Potential energy Match the following C D K Kinetic energy n Column I A V K n B If radius of nth orbit x EX x 2 x Z y ba Radius of nth orbit Angular momentum in lowest orbital p 0 9 1 2006 6M Column II S r 2 E A

Physical Chemistry
Atomic Structure1 163 167 3 168 2 171 2 172 3 175 3 176 4 179 3 180 4 183 3 184 4 187 4 188 3 169 4 173 2 177 3 181 4 185 3 170 2 174 3 178 1 1 4s 2 4p The shape of the orbital with I 3 4d a mon 1 Angular 2 Qantum nur 3 Dual nature 182 3 4 Black bod 186 2 200 In the plots the hydrog QUANTUM MECHANICAL MODEL OF ATOM 9 The designation of a subshell with n 4 and 3 is 4 4f peaks are 1 3 201 Which of for 1 y mus 2 y mu

Physical Chemistry
Atomic StructureFor H spectrum electron transition takes place from n 5 ton 2 then emitted wavelength of photon is 434 nm The wave length of photon in electron transition from n 4 to n 2 will be A 1 586 16 nm 31x8 2 48 608 nm 3 486 nm

Physical Chemistry
Atomic StructureAn incident UV light having wave length is incident on metal surface and the ejected electron has the velocity of V Another UV light having wave length is incident on the same metal surface ejects electron having velocity V then V2 V is 1 2hc 1 111 me2 2 2hc 2 2hc 1 1 me 2ho 2 A 2 2

Physical Chemistry
Atomic StructureFor which of the following sets of four quantum numbers an electron will have the highest energy I 1 3 2 4 3 4 2 2 1 To 1 2 1 2 1 2