General organic chemistry Questions and Answers

Organic Chemistry
General organic chemistrylion 1 5 Then rium 9 Major product of the given reaction is 1 8 Br K Br HBr Product 2 F Br Br

Organic Chemistry
General organic chemistryPartial reduction b Partial oxidation c Complete reduction d Complete oxidation 2 A Define electron gain enthalpy Explain why group 17 elements have large egative electron gain enthalpies and group 18 elements have high positive electron gain enthalpies 3 marks

Organic Chemistry
General organic chemistry70 Carbonyl compounds when treated with sodium bisulphite cc solution generally a crystalline sodium bisulphite addition product is formed but which of the following carbonyl compound not forms crystalline addition product a HCHO b CH3CHO Na HS c CH3COCH 3 71 Main product of the reaction is bu oby d C H5COC H5 all aldeh methyl ke

Organic Chemistry
General organic chemistry1 Among the given pairs in which pair first compound reacts faster than second compound in S SN1 reaction a CH3 CH2 CH CH2 Br or CH3 CH 2 CH CH3 THE Br CH 3 CH 3 b CH3 C CH CH or CH3 CH CH CH3 Br Br C Br 6 6 or CH 3 1 d CH3 C CH3 11 ve Br Br CH 3 or CH3 C CH 3

Organic Chemistry
General organic chemistryPlease solve the following 18 electron problem using two types of ionic and covalent models Ph P Ph OC OC OC RO Os BH RO Au Au co OR OR OR RIC W R CH O Co Co 8 Br CH t Bu Au t Au 1 CH3 Rh O Rh R

Organic Chemistry
General organic chemistryhich of the following pair s is are resonating structures e CH CH CH CH CH3 and CH CHCH CHCH e CH CH CH CH CH and CH CH CHCH CH CH CH CH and CH CHCH 15 CH CH CH CH CH and CH CH CH CH CH

Organic Chemistry
General organic chemistry49 How many terminal alkynes isomers are possible for the formula CeH10 O No 49

Organic Chemistry
General organic chemistryH of D H3C OH Which statement is correct SOCI SOCI Pyridine X y x and y are superimposable mirror images x and y are non superimposable mirror images O x and y are neither mirror images nor super imposable x and y are structural isomers

Organic Chemistry
General organic chemistryHow many of the following elimination takes place via E1CB mechanism Br OH H C CH CH C CH3OH H3C CH CH C CH3 MeO Ph O C H5O A NO 2 H MeO A H3C S CH CH OPh Ph NaOD D 0 A NO 2 01S H C S CH CH

Organic Chemistry
General organic chemistryC 1 In which of the following bond angles on sp hybridized are not contracted due to lone pair of electron 6 110 a OF b H O c CH3OCH3 3 R N d CH OH M 10 with ammonia and passed over

Organic Chemistry
General organic chemistry1 Compare relative stability of following resonating structures CH 0 CH CH C N a e CH 0 CH CH C N a a b c d b a c d b se CH O CH CH C N b CH O CH CH C N d c a d c b 6 d c d b a

Organic Chemistry
General organic chemistryNumber of hyperconjugation resonance structures of BH3 CO x Number of hyperconjugation resonance structures of BH3 PF3 Y Choose the correct relationship O x y 3 O O O x y 3 x y none of these

Organic Chemistry
General organic chemistryD Which of the following is most reactive toward S N2 Cl Cl a CH3 b c reaction wollot orb to ridw Cl Cl NO2 1 Cl naim in which pair first compound reacts faster than second compou

Organic Chemistry
General organic chemistry110 Vapours of an alcohol were passed over hot reduced copper On It gave an olefin The alcohol is a primary c tertiary b secondary d none of these 24 CU 2

Organic Chemistry
General organic chemistryWhich of the following species will not have a formal positive charge on a carbon atom OA The Intermediate that forms when tert butyl alcohol reacts with hydrochloric acid after loss of water OB Any Intermediate from reaction of acetyl chloride and methoxybenzene OC An aromatic cyclopropenyl compound OD The intermediate from reaction of NaSPh with 2 4 6 trinitrofluorobenzene

Organic Chemistry
General organic chemistryQ3 A How can we measure the aromaticity which of the following is aromatic put a circle 5 points H A Br H HO H or A H H B B H H B H B What is meant of 1 2 adduct and 1 4 adduct for the reaction of 1 3 butadiene with hydrobromic acid write the reaction 5 points OH H C Rank the following aromatic compound according to the order of reactivity of aromatic ring 5 points C H C H C H H D D Which of the following would be suitable to use when forming a Grignard reagent Put a circle 5 points NO NH CH D Br

Organic Chemistry
General organic chemistry99 A compound X when boiled with Na2CO3 solution gives glycol as the product What is X a Ethylene b Ethylene oxide c Ethyl bromide d Ethyl hydrogen sulphate CH CH tem Na CO3 2 On M OW

Organic Chemistry
General organic chemistry5Brst A of formula C3H6Cl2 on reaction with alkali can give B of formula C3H6O or C of formula C3H4 B on oxidation gave a compound of the formula C3H6O2 C with dilute H SO4 containing Hg 2 C3H6O which with bromine and NaOH gave the sodium ion gave D of formula salt of C H4O2 Then A is a CH3CH CHC12 CH3 C C b CH3CCl CH3 CH3 CH d CH c CH CICH CH Cl

Organic Chemistry
General organic chemistryFor zero order reaction which is correct relation between t1 2 and initial reactant concentration R o t1 2 R 0 t1 200 1 R o t1 2 R hr min t1 2 is independent of R o

Organic Chemistry
General organic chemistryResonance is not shown by Question Type Single Correct Type 1 2 3 4 O C CH CH CH CH

Organic Chemistry
General organic chemistryQ 33 Hydrocarbon P on heating produces 1 3 butadiene without any side products How many hydrogen atom s is are present in P Your Answer 8 Correct Answer 6 Rate this que

Organic Chemistry
General organic chemistrySome reactions are given below out of which the correct combination is Reactant Reagent Major Product 1 CH3CH CH Cl 500 C CH3CH CH CI 2 CH3CH CH HBr H2O2 CH3CH CH Br 3 CH3CH CH HBr 4 CH3CH CH HCI 1 2 3 CH3CH CH Br CH3CH CH CI

Organic Chemistry
General organic chemistryNegative hyperconugation 4 All of these 48 Which is aromatic a c 1 a b c 2 a b c d 3 a c d 4 b c d b H N d N N N N 48 4 B a c 1 a b c 2 a b c 3 a c d

Organic Chemistry
General organic chemistryn 3 show geometrical isomerism QNo 48 Which among the following compound show goomctrical isomerism O 3 Your Answer 4 Stotus incorrect CH CEC CH3 CH3 H2 Cl Br Complete Quest A

Organic Chemistry
General organic chemistry47 m directing character of Caused by 1 I effect 2 m effect 3 Negative hyperconugation 4 All of these 48 Which is aromatic C Cl is CI 47 48 1 I C 2 m 3 T 4 BUR

Organic Chemistry
General organic chemistryReactant CH3 KOH a CH3 OH CH3 OH a CH3 OH CH OH H SO Conc 1 NaH 2 CH 1 HBr 2 Mg 3 CH l 1 Na 14 2 CH p G r Products CH3 14 OCH3 CH3 CH3 CH3 OCH CH3 OCH

Organic Chemistry
General organic chemistryWhich of following is are correct Question Type Single Correct Type 1 2 3 H H H O is Aromatic O H H OH H H H O H OH H H Stability H O H

Organic Chemistry
General organic chemistryIdentify the true statement regarding which of these structure is a better representation of the real molecule and why A AJ N H H 0 N H These are equivalent resonance structures in terms of formal charges and electronegativities The structure on the right is favored because it has lower formal charges The structure on the left is favored because it has lower formal charges The structure on the right is favored because it puts the negative formal charge on the more electronegative atom The structure on the left is favored because it puts the negative formal charge on the more electronegative atom

Organic Chemistry
General organic chemistryU Why medicine bottles are mostly orange or in shades of brown Orange brown are the most commonly used colours because of their ability to prevent UV light from damaging or degrading any photosensitive contents that are prone to photochemical reactions 618 boml 99 E 92 E 50

Organic Chemistry
General organic chemistry4 Show how to make benzene from cyclohexene Show all the intermediates and reagents used 2 pts 5 Show how to accomplish the following conversion Show all the intermediates and reagents used 2 pts o

Organic Chemistry
General organic chemistry320 Which of the following is correct order i p CH CHO Ph CHO CH C CH Ph C Ph Reactivity in nucleophilic addition reaction NAR q Ph C Cl Ph Reactivity in hydrolysis CHO u CH CHO COCI COCI CH Ph C OR Ph C NH OCH CH Reactivity in nucleophilic addition reaction NAR COOH FO CHO COCI CN Reactivity in hydrolysis t HCHO CH CHO CH CO Ph CHO Reactivity towards Grignard reagent COOH Rate of decarboxylation 1 p q CHO NO 2 r s t COCI NO COOH

Organic Chemistry
General organic chemistry9 CH3 CH CH HBr CH3 Br 1 CH3 CH2 C CH3 1 CH3 CH3 2 CH3 CH CH CH Br CH3 3 CH3 CH C CHz 1 Br CH3 Br 4 CH3 CH CH CH3 CH3 perosade

Organic Chemistry
General organic chemistryWhich of the following is correct order of reactivity A B Vinyl chloride Altyl chloride Propyl chloride Propyl chloride Vinyl chloride Allyl chloride

Organic Chemistry
General organic chemistry21 Compound P on treatment with NaOH gives major product Q Predict the correct stereochemical descriptor for P and Q OTS coro P A P is R and Q is S B P is R and Q is Racemic C P is S and Q is R D P is S and Q is Racemic NaOH Q

Organic Chemistry
General organic chemistryWhat relationship can be used to find the mass of H O that can be produced from complete reaction of 3 moles of NaOH in the shown reaction 3 NaOH H3PO4 Na3PO4 3 H O a 3 mol H O X b 3 mol H O X c 3 mol NaOH X d 3 mol NaOH X e none correct OC Ob 40 00 g NaOH 3 mol NaOH Oe Od 3 NaOH 3 H20 3 H20 3 NaOH 3 H20 3 NaOH X X X X 1 mol H20 18 02 g H20 40 00 g NaOH 1 mol NaOH 1 mol NaOH 40 0 g NaOH 18 02 g H20 1 mol H20

Organic Chemistry
General organic chemistry5 CH CH CH 1 CH CH CH3 CH CH CH CH CH CH3 CH CH COOH CH3 CH CH CH CH3 OH i 6 CH3 CH CH C CH3 7 CH CHO CHO 1 CH3 CH CH CH3 8 9 CH3COOH CH CH CH

Organic Chemistry
General organic chemistry181 Which of the following undergoes dehydration readily a 1 phenyl 1 butanol L c 2 phenyl 2 butanol nd b 1 phenyl 2 butanol d 2 phenyl l butanol

Organic Chemistry
General organic chemistryIdentify the reagent used in the following transformation HO A B C D OH P LiAlH P NaBH MeOH P CeCl 7H O P HO P NaBH CeCl 7H O Q KMnO4 OH OH MnO CH Cl Q Ag CO on celite PhH Q MnO CH Cl Q OH

Organic Chemistry
General organic chemistryAmong the following pairs of orbitals which orbital will experience the larger effective nuclear charge i 2s and 3s ii 4d and 4f iii 3d and 3p a 4f 3d and 3s respectively b 2s 4d and 3p respectively c 2s 4d and 3d respectively d 4d 3p and 2s respectively

Organic Chemistry
General organic chemistryH2N CH2CH2CH CH2 CHO product is Only One Correct Answer B A H N CH CH CH CH2 CH OH C H Ni CN NH A O

Organic Chemistry
General organic chemistry13 Correct order of basic nature is 1 C H NH C H5NH C H 2N NH3 2 C H NH C H 3N C H NH NH3 3 C H NH C HyN CH NH NHg 4 NHg C H NH C H NH C HJgN

Organic Chemistry
General organic chemistrySerratia marcescens is a bacterial species that produces a red pigment when grown at 25 C and remains colorless at 37 C What is t O Mesophilic bacteria are adapting to a lower temperature The environment is expressing a phenotype A change in the genotype The protein being inactivated at high temperatures O Temperature rearranging the gene sequence

Organic Chemistry
General organic chemistryArrange each set of compounds in order of increasing boiling points CM3 Br 1 Bromomethane Bromoform Chloromethane Dibromomethane ii 1 Chloropropane Isopropyl chloride 1 Chlorobutane

Organic Chemistry
General organic chemistryAs it is directly proportional to no Of pi bond than why be nzene is in middle For the reactions 211 31 Select the correct option Question Type Single Correct Type 1 y z x 2 x z y AH x Kcal mol 1 AH y Kcal mol AH z Kcal mol 3 z x y 4 z y x

Organic Chemistry
General organic chemistryThe addition of a catalyst during a chemical reaction alters which of the following quantities Internal energy Enthalpy Activation energy Entropy

Organic Chemistry
General organic chemistryiv none of the above b The correct order of acidic strength among the followings II CH3CH OH III C H OH 1 H O i III IV II I iii IV III II I ii IV III I II iv I II IV III IV p chlorophenol
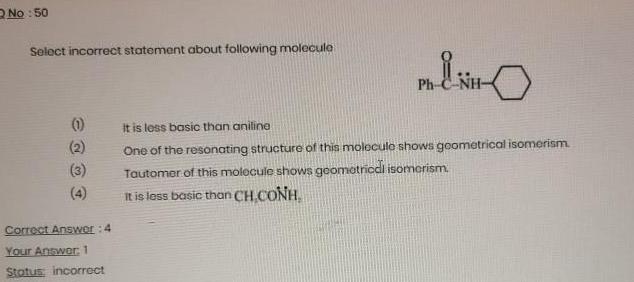
Organic Chemistry
General organic chemistryNo 50 Select incorrect statement about following molecule 1 Correct Answer 4 Your Answer 1 Status incorrect MENO It is less basic than aniline One of the resonating structure of this molecule shows geometrical isomerism Tautomer of this molecule shows geometrical isomerism It is less basic than CH CONH

Organic Chemistry
General organic chemistry4 In ethers the grou considered as a substituent in RH where a R is higher than R b R and R are same c R may be higehr than or same as R d R is smaller than R

Organic Chemistry
General organic chemistryWhich is the correct order of increasing basicity A CH3CH CH3 CH3CH SH CH3CH OH CH3CH2NH2 B CH3CH CH3 CH3CH OH CH3CH SH CH3CH NH2 C CH3CH NH2 CH3CH SH CH3CH OH CH3CH CH3 D CH3CH CH3 CH3CH OH CH3CH NH CH3CH SH

Organic Chemistry
General organic chemistryWhich of the following choices represent s a pair of resonance forms Mark all that apply 0 0 11 CH CH CH H H w propere OH CH CH CH H C CN H CH CH OCH CH CH OCH onance