Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
SolutionsQue 19 0 7g of a sample of Na2CO3 xH2O were dissolved in water and the volume was made to 100 ml 20 ml of this solution required 19 7 ml of N 10 HCI for complete neutralization The value of x is O 7
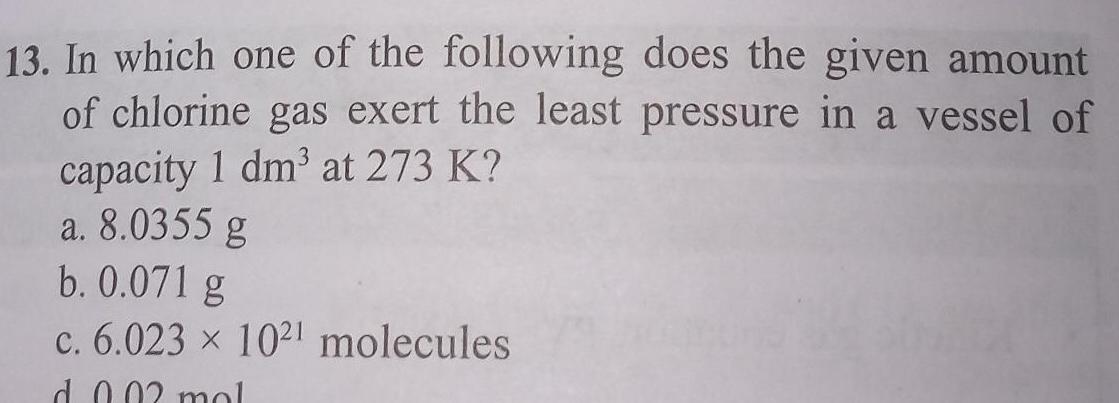
Physical Chemistry
General13 In which one of the following does the given amount of chlorine gas exert the least pressure in a vessel of capacity 1 dm at 273 K a 8 0355 g b 0 071 g c 6 023 1021 molecules d 0 02 mol

Physical Chemistry
Atomic Structure35 Five ionization energy values in kJ mol are listed below 1010 E 1290 E 376 E 870 E 830 E 1010 E These are a Successive ionization energies for the element with atomic number 5 b The first I E of successive elements in group 15 16 17 18 and 1 respectively c The first I E of elements with atomic number 1 to 5 d Successive L E for transition elements with four electrons in d subshell

Physical Chemistry
Atomic Structure14 If you are given Avogadro s number of atoms of a gas X If half of the atoms are converted into X energy AH The IE of X is by 2AH NA AH 2NA a C 5 The ene b 2NA AH d NA AH

Physical Chemistry
General5 The radii of two of first six Bohr s orbits of H atom are in the ratio 4 9 The energy difference between them may be a Either 12 09 eV or 3 4 eV b Either 2 55 eV or 10 2 eV c Either 1 89 eV or 0 49 eV d 1 89 eV only

Physical Chemistry
GeneralExpress your answer as signed integers separated by a comma View Available Hint s the oxidation states of V O 2 1 5 Your submission doesn t have the correct number of answers Answ No credit lost Try again

Physical Chemistry
GeneralA solution is made by adding 36 7 mL of pure alcohol to enough distilled water t make 75 0 mL of solution What is the percent v v of isopropyl alcohol in the sample a 48 9 c 95 8 b 51 1 d 4 2 e 50 0

Physical Chemistry
Electrochemistry6 Match the standard reduction potentials for each half reaction and the electric potential for the voltaic cell Given half cell Be Be reduction half reaction Be 2e Be half cell Hg Hg reduction half reaction Hg 2e Hg anode Be cathode Hg

Physical Chemistry
GeneralA student dissolved 0 515 mol of magnesium chloride in enough water to make 225 mL of solution What is the molar concentration of the magnesium chloride in solution e 0 44 mol L a 2 29 mol L b 0 00229 mol L c 0 00458 mol L d 4 58 mol L

Physical Chemistry
Solutionsd 15 75 24 The equivalent conductivity of monobasic acid at infinite dilution is 348 ohm 1 cm eq If the resistivity of the solution containing 15 g acid molar mass 49 in 1 litre is 18 5 ohm cm what is the degree of dissociation of acid a 45 9 c 60 4 b 40 2 d 50 7

Physical Chemistry
GeneralReason At any gas oc 9 Assertion The number of significant figures in 1502 cm is two Reason In 1502cm zero may or may not have any significance

Physical Chemistry
Atomic Structure25 An electron in an atom undergoes transition in such a way that its kinetic energy changes from x to the change in potential energy will be 4 3 a x 2 3 C X 4 b 3 X 8 3 d x 4

Physical Chemistry
Atomic StructureThe nucleus of an atom is located at x y z 0 If the probability of finding an s orbital electron in a tiny volume around x P y z 0 is 7 10 4 The probability of finding of electron in the same sized volume around z P x y 0 14 x 10 4 7 x 10 14 7 x 10 4

Physical Chemistry
Solid stateIn a spinel structure AB204 the divalent cations A occupy x of the tetrahedral voids while trivalent cations B occupy y of the octahedral voids and z of the tetrahedral voids Then the value of y z x is Answer 00 01 02 03 04 05 06 07 8 09

Physical Chemistry
General5 A wagon loaded with iron blocks is pushed up an inclined plane to its highest point The total mass of the wagon is 50 kg and the height of the topmost point from the ground is 10 meters What is the total potential energy of the wagon at the top Show formulae and working part clearly

Physical Chemistry
Chemical Bonding31 Two samples X and Y contain equal amount of radioactive substances If of the sample X and 1th of the sample Y remain after 8 hours then 16 256 the ratio of half life periods of X and Y is a 2 1 b 1 2 c 1 4 d 4 1 32 The decay constant 41

Physical Chemistry
General5 Which of the following factors decreases the overpotential a increase in thermionic work function c increase in current density S What is the condition under which O motal inn i b smooth surface d increase in temperature 10 hydron

Physical Chemistry
Atomic Structure34 The radius of the first orbit of hydrogen atom is 0 52 x 10 8 cm The radius of the first orbit of Het ion is a 0 26 10 8 cm c 1 04 x 10 8 cm b 0 52 x 10 8 cm d 2 08 x 10 8 cm 35 The ratio of ionigati

Physical Chemistry
SolutionsThe vapour pressure of water at 20 C is 17 54 mm When 20g of a non ionic substance is dissolved in 100g of water the vapour pressure is lowered by 0 30 mm What is the molecular UPSEAT 2001 weight of the substances a 210 2 c 215 2 b d 206 88 200 8 our pressure

Physical Chemistry
GeneralThe concentration of Hg2 is controlled by equilibrium with a mineral hase The water in the sediments is characterized by pH 7 3 PH2S 10 5 0 bar Pco2 10 22 bar and CI 10 3 2 M a Which mercury Hg2 solid will control the solubility of Hg2 Use the appropriate Hg2 solid species from the following list and Show your calculations Hg OH s Hg 2 OH HgO s H O Hg 2 OH Hg CN s Hg 2 CN HgCO3 s Hg2 CO3 HgS s Hg S Kso 10 25 40 K 10 25 55 Kso 10 39 28 Kso 10 22 52 Kso 10 52 01

Physical Chemistry
Atomic Structurea 0 002 b 0 1 c 0 5 21 The ionisation energy of He is 19 6x 10 energy of the first stationary of Li2 is a 2 2 x 10 15 J atom b 8 82 x 10 17 J atom c 4 41 10 16 J atom 1 d 4 41 x 10 17 J atom d 0 7 J atom The urface

Physical Chemistry
General16 mL of a hydrocarbon gas was exploded with exc ess of oxygen On cooling the volume of the resulti ng gaseous mixture was reduced by 48 mL When K OH was added there was a further decrease of 48 mL in the volume Find the molecular formula of th

Physical Chemistry
GeneralWhat can happen if too much solvent is added to the sample A Nothing it is fine to add excess solvent B The solute might remain dissolved C The solution will have to be cooled much quicker D Activated charcoal can dissolve in it

Physical Chemistry
Gaseous and liquid statesA metal oxide has the formula M 03 This oxide reacts with hydrogen to produce M and H O according to the reaction unbalanced M O3 H M H O If 32 g of M 03 required 1 2 g of hydrogen for complete reduction The atomic mass of metal M is in gm mol

Physical Chemistry
GeneralShow how to carry out the transformation in the highest yield possible Select the appropriate reagents and draw the correct organic product at each step Identify reagent 1 1 Zn HCI 2 NaOH HNO3 H SO4 Br FeBr3 Identify reagent 2 HNO H SO reagent 1 product 1 HNO3 H SO4 1 Zn HC1 2 NaOH reagent 2 Draw product 1 Select Draw Rings G C Br More Erase Q2 Q

Physical Chemistry
Solutions33 An aqueous solution of N gas obeys Henry s law at very low concentration of gas in wate nN nwater How many moles of N gas will dissolve in 18 kg water at 27 C and 1 6 107 Pa KH Henry s law constant for N gas in water at 27 C 80 K bar ID Q 529195 Correct answer is 2

Physical Chemistry
GeneralSO3 can be prepared by the following sequence of reactions Sg 8 02 8 SO2 50 yield Reaction I 2 SO2 02 2 SO3 100 yield Reaction II A sample containing 50 by mass of each Sg and O2 is taken in the initial reaction mixture If the sum of weights of reactants initially taken to obtain 320 g of SO3 is x g for reaction I then value of is X 16 yield of reactions are mentioned

Physical Chemistry
Atomic Structure39 The uncertainty in the velocity of particle of mass 6 626x10 28 Kg is 10 m sec What is the uncertainty in its position in nm a 1 2 b 2 5 c 4 TC d YAT 4

Physical Chemistry
GeneralThe spinel structure consists of an array of 0 ions in fcc arrangement General formula of spinel is AB 04 Cations of A occupy 1 8 th of the tetrahedral voids and cations of B occupy half of the octahedral voids Spinels are always electrically neutral and these are important class of compounds There are also inverse spinels in which the distribution is B AB 04 Those in square brackets denote the species in octahedral voids Question 18 Which of the following is an inverse spinel Options a PbFe 04 b ZnO Al O3 c Fe304 d Mg0 Al O3

Physical Chemistry
GeneralA mixture of C3H8 and oxygen in 1 L closed vessel has an internal pressure of 4 atm at 100 C When the mixture is ignited the reaction produces CO g and H O g until all the oxygen is consumed After the reaction pressure of the vessel is 4 2 atm at the same temperature The mass of oxygen present before the reaction is L atm mol K R 0 082

Physical Chemistry
SolutionsEXAMPLE 2 25 If the radiator of an automobile contains 12 L of water how much would the freezing point be lowered by the addition of 5 kg of prestone glycol C H4 OH How many kg of Zeron methyl alcohol would be required to produce the same result

Physical Chemistry
Chemical kineticsIt can be determined graphically by drawing a tangent at time ton either of the curves for concentration of R and P vs time t and calculating its slope Fig 4 1 So in problem 4 1 at 600s for example can be calculated by plotting concentration of butyl chloride as a function of time A tangent is drawn that touches the curve at t 600 s Fig 4 2 The slope of this tangent gives the instantaneous rate So instat 600 s At t 250 s t 350 s t 450 s 0 0165 0 037 800 400 s mol L 5 12 x 10 mal L s Finst 1 22 x 10 mol L s Finst 1 0 x10 mol L s 6 4 x 105 mol L g inst Now consider a reaction Hg l Cl g HgCl s Where stoichiometric coefficients of the reactants and products are same then rate of the reaction is given as A Hg A C1 A HgCl At At At Rate of reaction H nce of any of the reactants is same as the rate reaction two moles of

Physical Chemistry
GeneralWhen crystals of potassium chloride is heated with Mn2O3 mixedoxide and conc H SO4 then ID Q 529182 Options are A Cl is produced B Its redox change C Resulting mixture after dilution by water gives buff colour ppt with H S D Resulting mixture after dilution by water gives ppt with excess KOH

Physical Chemistry
Solid stateQuestion 25 Which is are correct statement about zinc blende structure Options a The number of first neighbors of S is 4 b The maximum distance between Zn is a3 where a edge length of unit cell a 3 c If all tetrahedral voids occupied by Zn then C N of S is 8 d If all tetrahedral voids occupied by Zn then C N change from 4 4 to 8 8

Physical Chemistry
Solutions4 go 24 An element X of atomic mass 25 0 exists as X i benzene to the extent of 100 When 10 30 saturated solution of X in benzene is added to 20 0 g of benzene the depression in freezing point o the resulting solution is 0 51 K If K for benzene is 5 1 Kkgmol the solubility of X in 100 g d benzene will be 1 2 9 g 2 3 0 g 3 0 7 g 4 0 3 g R S asr 30 19 sw To Iniog anilin SU xaja c

Physical Chemistry
Generals p t Cs D q t 44 How many moles of ferric alum NH SO Fe SO 24H O can be made from the sample of Fe containing 0 0056 g of it a 10 mol b 0 5 x 10 mol c 0 33 10 mol d 2 104 mol

Physical Chemistry
EquilibriumA 1 025 g sample containing a weak acid HX molecular weight 82 g mot is dissolved in 60 mL water and titrated with 0 25 M NaOH When half of the acid was neutralised the pH was found to be 5 0 At the equivalence point the pH was 9 0 The weight percentage purity of HX in the sample is Correct Answer 80

Physical Chemistry
EnergeticsOne mole of a gas changed from its initial state 15L 2 atm to final state 4L 10atm reversibly If this change can be represented by a straight line in P V curve maximum temperature approximate the gas attained is x K Then find the value of x x 700 value is in hundreds

Physical Chemistry
EnergeticsFor the reaction CO g O2 g CO g AH 67650 cal at 25 C Calculate AH at 100 C given that the required molar heat capacities are as follows C CO g 6 97 cal C C CO g 8 97 cal C Cp O2 g 7 00 cal C 1 54 6 cal 3 67681 1 cal 2 67650 4 cal 67762 5 cal

Physical Chemistry
Solutions2 What will be the molarity of a solution which contains 5 85 g of NaCl s per 500 mL a 4 mol L 1 c 0 2 mol L 3 If 500 ml b 20 mol L 1 d 2 mol L

Physical Chemistry
General36 3 68 of a mixture of CaCO and MgCO is heated to liberate 0 04 mole of CO The mole of CaCO and MgCO in the mixture is respectively a 50 50 b 60 40 c 40 60 d 30 70

Physical Chemistry
ElectrochemistryThe molar conductivities of Mg HCOO 2 MgCl2 and HCI are 230 280 and 425 Scm mol1 respectively Calculate molar conductivity of HCOOH Question Type Single Correct Type 1 2 3 4 400 Scm mol 1 80 Scm mol 1 40 Scm mol 1 160 Scm mol 1

Physical Chemistry
Solutionsc 20 30 d 30 20 7 Concentrated aqueous sulphuric acid is 98 H SO by mass and has a density of 1 80 g mL Volume of acid required to make one litre of 0 1 M H SO solution is a 11 10 mL b 16 65 mL c 22 20 mL d 5 55 mL

Physical Chemistry
Solutions42 Which of the following molarity values of ions in a aqueous solution of 5 85 w v NaCl 5 55 w v CaCl and 6 w v NaOH are correct Na 23 Cl 35 5 Ca 40 16 a CH 2M c Ca2 0 5 M b OH 1 5 M d All of these 48

Physical Chemistry
General5 Assertion A certain element X forms three binary compounds with Chlorine containing 59 68 68 95 and 74 75 Chlorine respectively These data illustrate the law of multiple proportions Reason According to law of multiple proportions the relative amounts of an element combining with some fixed amount of a second element in a series of compounds are the ratios of small whole numbers

Physical Chemistry
Generald 6 63 x 10 2 m P 43 If E E and E represents the kinetic energy of an electron a particle and proton respectively each moving with same de broglie wavelength then a E E E e P c E E E P a a b E E E d E E E P e a

Physical Chemistry
Chemical kineticsFor a reaction A g Products the half life was found to be 20 minutes when the initial pressure of A was 0 5 atm If the time needed for the pressure of A to become half starting from 1 atm is 14 14 minutes then what is the order of the reaction a Order 1 b Order 0 c Order 1 5 d Order 0 5

Physical Chemistry
Generala 66 6 c 25 b 75 d 33 3 38 If 42 g of an unknown gas X occupies a volume of 125 L at 0 3 bar pressure and 300 K temperature then the gas X could be a N C CO b CO d NO 45

Physical Chemistry
Chemical kinetics26 In an evacuated vessel of 8 2 L capacity 0 2 moles of A g is taken vessel is sealed and heated to 127 C by which the following first order reaction occurred at constant volume and temperature A g 2 B g C s The vapour presure of C s is 0 1 atm at 127 C If the half life of reaction is 20 min then the correct information s related with reaction is are R 0 082 L atm K mol ID Q 529175 Options are A Ptotal 1 7 atm att B Ptotal 0 98 atm after 10 decomposition of A g C Ptotal 0 88 atm after 5 decompositon of A g D Ptotal 1 3 atm at t 20 min

Physical Chemistry
Surface chemistryLE 2 21 CNS ions give red colour with Fe3 ions in aqueous solution as Fe aq 3CNS aq Fe CNS 3 aq red If 0 1 M KCNS solution is separated from 0 1 M FeCl solution by means of a semi permeable membrane red colour will appear on a FeCl solution c Both sides 3 b KCNS solution side d Neither side