Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
General11 Which of the following statements about a compound is incorrect a A molecule of a compound has atoms of different elements b A compound cannot be separated into its constituent elements by physical methods of separation c A compound retains the physical properties of its constituent elements d The ratio of atoms of different elements in a compound is fixed

Physical Chemistry
SolutionsThe Kip of Cd OH 2 in 7 2x10 15 Cd2 aq 2 e Cd s 0 40 V Part A Calculate the standard electrode potential for half reaction Ca OH 2 0 2e Cd a 2OH aq Express your answer to two decimal places and include the appropriate units A 0 82 V

Physical Chemistry
GeneralTwo radioactive nuclides A and B have half life 100 min and 50 min respectively A fresh sample contains nuclides of B to be 16 times that of A The time required by the nuclide A to become 4 times that of B is x hours Find the value of X lin 5

Physical Chemistry
GeneralIn the estimation of sulphur organic compound on treating with conc HNO is converted to 1 SO 2 H S 3 H SO 4 SO Calculate the number of atoms in each of the following 57 moles of Ar in 57 of he iii 52 g of He NA 6022 10

Physical Chemistry
SolutionsEXAMPLE 2 22 At 17 C the osmotic pressure of sugar solution is 580 torr The solution is diluted and the temperature is raised to 57 C when the osmotic pressure is found to be 165 torr The exten of dilution is a 2 times b 3 times c 4 times d 5 times

Physical Chemistry
General5 A 25 0 mm x 40 0 mm piece of gold foil is 0 25 mm thick The density of gold is 19 32 g cm How many gold atoms are in the sheet Atomic weight Au 197 0 A 7 7 x 10 3 C 4 3 10 1 B 1 5 x 1023 D 1 47 x 10

Physical Chemistry
GeneralWhich of the following properties don t help in differentiating different hydrated isomers of CrCl 6H 0 1 Conductivity measurements 3 Dipole moment 2 Precipitation by AgNO 4 Magnetic moment

Physical Chemistry
General4 The number of atoms present in one mole of an element is equal to Avogadro number Which of the following element contains the greatest number of atoms a 4 g He b 46 g Na c 0 40 g Ca d 12 g He 5 If the

Physical Chemistry
ElectrochemistryWrite the Nernst equation for the following half reaction and find E when pH 3 00 and PAH 1 00 mbar As s 3H 3e ASH g E 0 238 V A 0 356 V B 0 556 V C 0 356 V D 0 556 V Answer A

Physical Chemistry
Atomic StructureThe ionization energy of He atom in the ground state may be a 13 6 eV b 54 4 eV c 108 8 eV The binding energy for the third electron in the ground d 27 0

Physical Chemistry
EquilibriumQ Please help me with the following MC Thnx 1 When solid aluminum sulfate is in equilibrium with its ions the ratio of aluminum ions to sulfate ions is a 1 1 d 2 1 b 1 2 e 2 3 c 1 3 f 3 1 2 Which of the following cannot act as a Brons

Physical Chemistry
General23 1 g sample of alkaline earth metal react completely with 4 08 g H SO and yields an ionic product MSO Then find out the atomic mass of alkaline earth metal M a 9 b 24 c 40 d 87 32

Physical Chemistry
Generalmich of the following molecules would have the following potential energy diagramfor rotation about its C2 C3 bon Energy kcal mol 0 0 9 180 butane 5 0 1 8 2 methylbutane 2 3 dimethylbutane 5 0 O torsional angle 0 9 60 4 2 120 09 180

Physical Chemistry
Equilibrium15 The equivalent conductivity of KCl at infinite dilution is 130 mho cm eq The transport number of Clion in 2 KCl at the same temperature is 0 505 The transport number of K ion is La 0 495 4 x 1 1 cl tk c 0 0495 b 0 505 d cannot be predicted In the problem 15 ionic conductance of K ion is a 64 35 b 60 20 c 262 26 d 26 22

Physical Chemistry
GeneralA tetra atomic molecule X on reaction with nitrogen oxide Oxidation State 1 produces two substances Y and Z y is a dehydrating agent while compound Z is a diatomic gas which shows almost inert gas behavior The substances X Y and Z are 1 P N O O 2 P P O Ar 3 P PO O 4 P P O N Arrange the following structure according to their increasing order of acidic behavior in nolar solvent
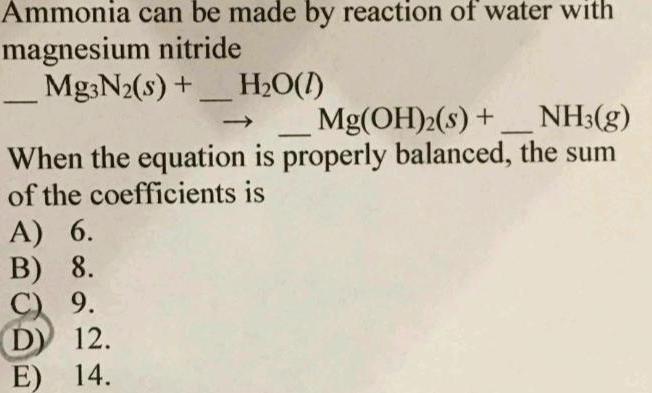
Physical Chemistry
GeneralAmmonia can be made by reaction of water with magnesium nitride Mg3N s H O 1 Mg OH s NH3 g When the equation is properly balanced the sum of the coefficients is A 6 B 8 C 9 D 12 E 14

Physical Chemistry
Chemical kineticsThe reaction rate is defined as the rate at which the concentration of the reactants with time or the concentration of products with time O Increase increase O Decrease decrease O Decrease increase O Increase decrease

Physical Chemistry
EnergeticsFor an exothermic reaction following two steps are involved Step 1 A B 1 slow Step 2 1 AB fast Which of the following graphs correctly represent this reaction 3 Potential energy Potential energy A B Reaction coordinate a A B AB Reaction coordinate c AB 2 Potential energy A B Potential energy Reaction coordinate b A B Reaction coordinate d AD AB

Physical Chemistry
GeneralThe products of the following I and II sequences are related as H3C C C CH3 A Diastereomers II 1 H Pd BaSO4 2 Br CCl4 1 Br CCl4 1 eq 2 H Pd B Identical C Enantiomers D Geometrical is

Physical Chemistry
EnergeticsQ For a reaction ALP the plots of A and P with time at temperatures T and T are given below 10 A mol L Time T 2 T 10 A P mol L If T2 T1 the correct statement s is are Assume AH and AS are independent of temperature and ratio of In K at T to ln K at T2 is greater than 1 T2 TT Here H S G and K are enthalpy entropy Gibbs energy and equilibrium constant respectively A AH 0 AS 0 B AGO 0 AH 0 C AGO 0 AS 0 D Time FF T T LIFE Adu 20181
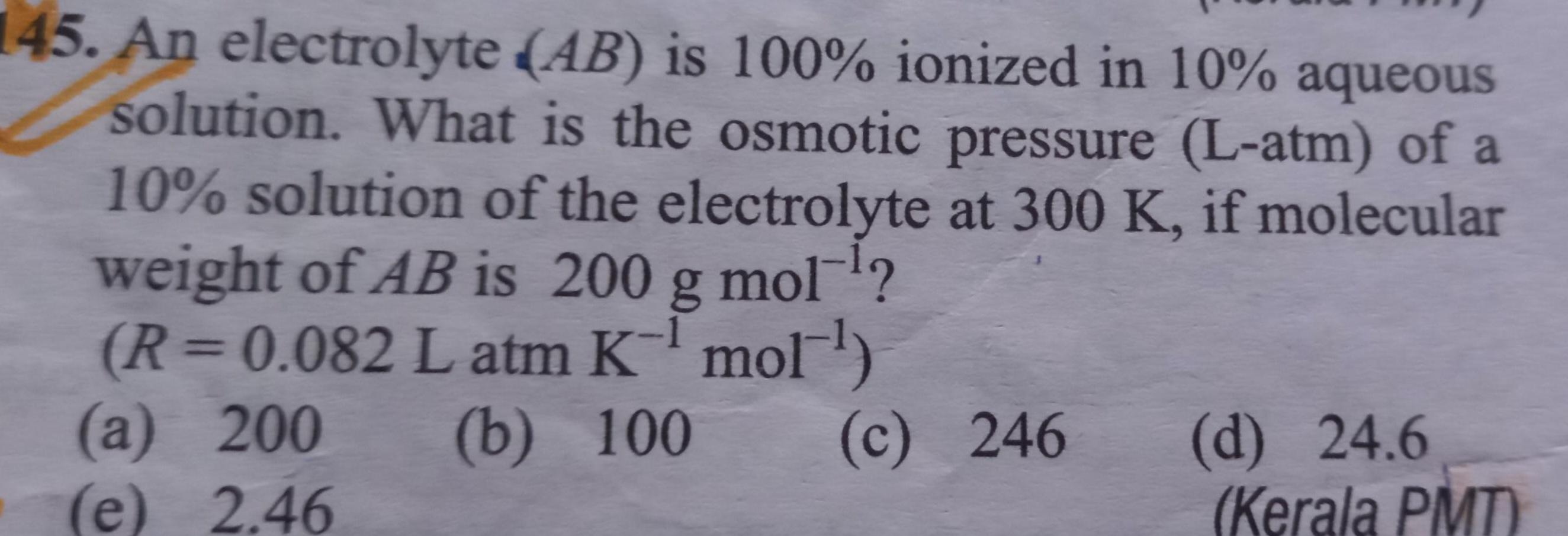
Physical Chemistry
Chemical kinetics145 An electrolyte AB is 100 ionized in 10 aqueous solution What is the osmotic pressure L atm of a 10 solution of the electrolyte at 300 K if molecular weight of AB is 200 g mol R 0 082 L atm K mol b 100 a 200 2 46 c 246 e d 24 6 Kerala PMT

Physical Chemistry
General1 18 4 A salt M4 ionizes as MAM 2A It was found that a given solution of the salt had the same freezing point as solution of glucose of twice the molality The apparent degree of ionization of the salt is 2 0 33 3 0 50 2 9 2 3 4 6 4 2 3 1 0 25 4 0 67 The solubility product of declis 18x10 19 Precenitation of doct will occur only when equa

Physical Chemistry
GeneralA certain half reaction has a standard reduction potential Ered 1 12 V An engineer proposes using this half reaction at the anode of a galvanic cell that must provide at least 1 50 V of electrica

Physical Chemistry
GeneralConsider the phase diagram shown What is the normal freezing point Pressure not to scale 72 9 atm 5 1 atm atm 31 C 0 C SOLID 78 5 C 100 C 56 7 C LIQUID GAS 78 5 C 56 7 C Temperature not to scale 31 C

Physical Chemistry
Atomic Structurec AR AR 1 d 2 1 96 Ionization potential of hydrogen atom is 13 6 eV Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy 12 1 eV According to Bohr s theory the spectral lines emitted by hydrogen will be a one c three b two d four last line is tot a c 100 The a b

Physical Chemistry
Chemical Bonding16 2 gram moles of H and 5 88 gram moles of 12 are heated at 444 C At equilibrium 11 28 gram moles of HI are formed Calculated the equilibrium constant K for the reaction H g 12 g 2HI g Ans 50 2

Physical Chemistry
General276 62 If an electron is revolving in its Bohr orbit having 73 I Bohr radius of 0 529 A then the radius of third orbit is C a 4234 nm c 4 761 b 4496 d 5125 nm re L

Physical Chemistry
EquilibriumFor the equilibrium N2 g 3H g 2NH3 g the equilibrium constant K is expressed as Kp A B Solution Kp D C Kp Kp 3 PN2 PH Kp N2 3H22NH3 NH PN2 Kp X 2 P NH3 PNH3 3 P N P NH3 3 PN PH PN X 3pN PNH P NH3 4 3 X PN2 P NH3 4 33 X PN2 P NH3 3 32 3 P N2 4 X PN2 3PN PH

Physical Chemistry
Chemical BondingCompound P forms a precipitate with AgNO The precipitate dissolves in excess reagent P P cannot be 1 KOH 2 KCN 3 Na S O 4 NH Addition of sodium hydroxide solution to a weak acid HA results in a buffer of pH 6 If ionization

Physical Chemistry
Generalc 75 mL d 500 mL 9 In an organic compound of molar mass 108 g mol C H and N atoms are present in 9 1 3 5 by weight Molecular formula can be a C H N c C H N b C H N d C H N 28

Physical Chemistry
GeneralA certain solution of 1 m benzoic acid in benzene has a freezing point of 3 1 C and a normal boiling point of 82 6 C The freezing point of benzene is 5 5 C and its boiling point is 80 1 C Analyze the state of the solute benzoic acid at two temperatures and comment

Physical Chemistry
General10 A 2B 3C AB C 2 Reaction of 6 g of A 6 1023 atoms of B 0 036 mole of C yields 4 8 g of compound AB C If the atomic masses of A C are 60 80 amu respectively the atomic mass of B is a 60 amu c 90 amu L b 50 amu d 120 amu

Physical Chemistry
General62 On analysis a certain compound was found to contain iodine and oxygen in the ratio of 254 g of iodine at mass 127 and 80 g oxygen at mass 16 What is the formula of compound a IO b 1 0 d 1 0 c 1 0 63 0 5 mol of potassium ferrocyanide com to Formula

Physical Chemistry
EquilibriumThe solubility product of AgCl is 1 8x10 Precepitation of AgCl will occur only when eq volumes of which of the following solutions are mixed 1 10 M Ag and 10 M CI 2 10 M Ag and 10 MCI 4 10 M Ag and 10 M CI 3 10 M Ag and 10 M CI

Physical Chemistry
EquilibriumAn empty steel vessel is charged with 0 430 atm of C and O 430 atm of A Once the system reaches equilibrium according to the reaction below what is the equilibrium partial pressure of C Kp for this reaction is 11 2 A g B g C g

Physical Chemistry
General3 9 Calculate velocity of an electron present in the third orbit of the hydrogen atom Also calcula number of revolutions per second that this electron makes around the nucleus Velocity of electron in 3rd orbit

Physical Chemistry
Chemical BondingConsider the reaction Cl2 aq H S aq S s 2H aq 2Cl aq The rate equation for this reaction is rate K Cl H S which of these mechanism is consistent with this rate equation 1 Cl H S H CI CI HS slow CI HS H CI S fast II H S H HS fast equilibrium Cl HS 2CI H S slow Question Type Single Correct Type 1 2 I only II only

Physical Chemistry
Chemical kineticsA and B decompose via first order kinetics with half lives 54 0 min and 18 0 min respectively Starting from a equimolar non reactive mixture of A and B the time taken for the concentration of A to become 16 time that of B is min Round off t the Nearest Integer

Physical Chemistry
EquilibriumCH3COOC H5 H 0 CH3COOH C H5OH Ans 4 17 75 gram of PC15 is heated in a closed vessel of one litre at 580 K Chlorine formed at equilibrium is 3 337 g Calculate K for the reaction PC15 8 Cl g PC13 g Ans 0 058 mol L hemical equilibrium reaction

Physical Chemistry
EquilibriumIn the heterogeneous chemical equilibrium reaction NH4 2S g 2NH3 g H S g the total pressure of the system at constant temperature and at equilibrium is 1 2 atmosphere Calculate Kp for the reaction Ans 0 256 atm un moles of PC are heated at 600 K in a closed vessel of 2 litre At equilibrium 40 PC15 PCL Clofg

Physical Chemistry
Solutions17 What is the pH of the solution that results when 0 093 g of Mg OH 2 is mixed with a 75 0 mL of 0 0500 M HCl b 100 0 mL of 0 0500 M HCl c 15 0 mL of 0 0500 M HCI d 30 0 mL of 0 0500 M MgCl

Physical Chemistry
Generalmon and 59 If 13 6 eV energy is required to separate a hydrogen atom into a proton and an electron then the orbital radius of electron in a hydrogen atom is a 5 3 x 10 m c 6 3 x 10 m ne gets as b 4 3 x 10 m d 7 3 x 10 m

Physical Chemistry
Surface chemistry3 Two students X and Y report the mass of the same substance as 7 0 g and 7 00 g respectively which of the following statement is correct a Both are equally accurate b X is more accurate than Y c Y is more accurate than X d Both are inaccurate scientifically

Physical Chemistry
Generald 23 u 17 Insulin contains 3 4 sulphur by mass What will be the minimum molecular weight of insulin a 94 117 u b 1884 u c 941 u d 976 u 18 A 100 g of a sample of haemoglobin on analysis was

Physical Chemistry
Generalexcited state 57 In which of the following Bohr s orbit n a hydrogen atom emits the photons of lowest frequency a n 2 tom 1 state en 3 state b 4 tom 2 c n 4 tom 1 d n 4 ton 3 lectron and

Physical Chemistry
Equilibrium100 ML of 1 2M FeCl3 solution is mixed with 200ml of 1 5M Mg Cl and the resulting solution is dilute to 500ml Then FeCl Fe 3Cl MgCl Mg 2CH A The molarity of Fe in the resulting solution is 0 24 M B The molarity of Mg2 ion in the resulting solution is 0 6M C The molarity of CI D The molarity of CI ion in the resulting solution is 1 92 M ion in the resulting solution is 3 2 M

Physical Chemistry
Equilibrium8 For the reversible equilibrium reaction A 2B 3C 4D the rate constant for the forward and backward reaction are 8 15 x 10 5 respectively Calculate K sible reaction e 2 38 x 10 and Ans 2 920

Physical Chemistry
Gaseous and liquid states50 ml of gas A diffuses through a membrane in the same time as for the diffusion of 40 ml of a gas B under identical conditions of temperature and pressure If the molecular weight of A is 64 the molecular weight of B is

Physical Chemistry
SolutionsWhat is the molarity of SO ion in aqueous solution that contain 34 2 ppm of Al2 SO4 3 Assume complete dissociation and density of solution 1g mL A 3 10 M C 10 M B 2 10 M D None of these

Physical Chemistry
Atomic Structure4 0 g of caustic soda NaOH mol mass 40 contains same number of sodium ions as are present in a 10 6 g of Na CO mol mass 106 b 58 5 g of NaCl Formula mass 58 5 c 100 ml of 0 5 M Na SO4 Formula mass 142 d 1mol of NaNO mol mass 85