Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Generald 35 5 g 11 4 4 g of an oxide of nitrogen gives 2 24 L of nitrogen and 60 g of another oxide of nitrogen gives 22 4 L o nitrogen at S T P The data illustrates a Law of conservation of mass b Law of constant proportions c Law of multiple proportions d Law of reciprocal proportions

Physical Chemistry
GeneralFollowing questions No 3 11 are multiple choice questions carrying I mark each Read the following statements and identify incorrect statements Carbocation behave as nucleophile In homolytic bond cleavage free radical is formed c Ethoxyethane is functional group isomer of ethanol d In ethyne two sigma and two pi bonds are present X i i iv ii i ii iii ii iv iv iii iv

Physical Chemistry
Nuclear chemistryA radioactive material has mean lives of 1620 years and 660 years for a and emissions respectively The material decays by simultaneous a and emission The time in which of the 4 material remains intact is 1 4675 years 2 720 years 3 650 years 4 324 years

Physical Chemistry
General32 Out of 1 0 g dioxygen 1 0 g atomic oxygen and 1 0 g ozone the maximum number of oxygen atoms are contained in a 1 0 g of atomic oxygen b 1 0 g of ozone c 1 0 g of oxygen gas d All contain same number of atoms 33 The maximum volume at STP is occupied by 42 4

Physical Chemistry
General1 18 5 V 2 13 5 V 3 11 5 V 4 6 5 V A capacitor of capacitance 100 F is charged by connecting it to a battery of EMF 12V and internal resistance 252 The time taken before 99 of the maximum charge is stored on the capacitor 1 0 92 ms 2 0 4 ms 3 0 8 ms 4 0 1 ms

Physical Chemistry
Electrochemistryc 0 0133 M 11 0 04 N solution of a weak acid has conductivity 4 23x10 mho cm 1 If the degree of dissociation of acid at this dilution is 0 0612 then equivalent 2 conductivity at infinite dilution is mho cm eq b 180 a 172 8 c 190 d 160 onductance of H and CH3COO at

Physical Chemistry
Atomic Structure10 Two elements X and Y combine in gaseous state to form XY in the ratio 1 35 5 by mass The mass of Y that will be required to react with 2 g of X is b 3 55 g a 7 1 g c 71 g d 35 5 g

Physical Chemistry
Electrochemistry4 0 001174 g 5 amp of current is passes for 193sec through on aqueous solution NaCl the total no of a equivalent of NaOH formed in the solution assuming 60 current efficiency for this process is 1 0 006 3 0 003 2 0 01 4 None

Physical Chemistry
ElectrochemistryAt 291 K the molar conductivity at infinite dilution of NH4Cl NaOH NaCl are 129 8 217 4 108 9 ohm cm mol respectively If the molar conductivity of centinormal solution of NH4OH is 9 33 ohm cm mol 1 what is the percentage dissociation of NH4OH at th dilution A B 0 392 39 2 3 92

Physical Chemistry
Atomic StructureWhat minimum number of atoms ions should be present in a sample of H like species so that a maximum of 6 spectral lines can be produced of electronic transition from fifth excited state upto n 2 2

Physical Chemistry
Chemical BondingFor the given real gas reaction 2A g B g D g carried out in 10 litre rigid vessel the initial pressure is 50 bar which decreases to 20 bar in the course of reaction If heat liberated in the reaction is 400 kJ then what is change in magnitude of internal energy of the reaction in KJ Fill your answer as sum of digits excluding decimal places till you get the single digit answer

Physical Chemistry
Chemical Bonding9 At 88 C benzene has a vapour pressure of 900 torr and toluene has a vapour pressure of 360 torr What is the mole fraction of benzene in the mixture with toluene tha will boil at 88 C at 1 atm pressure benzene toluene for an ideal solution a 0 416 c 0 688 b 0 588 d 0 740
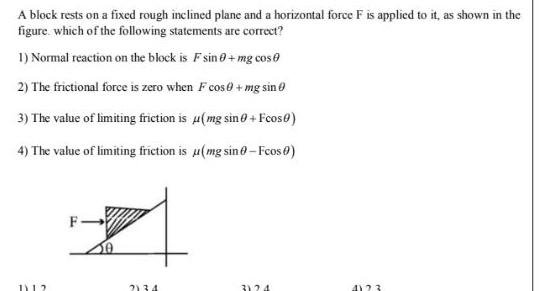
Physical Chemistry
GeneralA block rests on a fixed rough inclined plane and a horizontal force F is applied to it as shown in the figure which of the following statements are correct 1 Normal reaction on the block is F sin 0 mg cos 2 The frictional force is zero when F cos mg sin 3 The value of limiting friction is u mg sin 0 Fcos0 4 The value of limiting friction is u mg sino Fcos 0 1112 F 2134 3124 4123

Physical Chemistry
GeneralPHYSICS We have three beakers A B and C containing glycerin water and kerosene respectively They an stirred vigorously and placed on a table The liquid which comes to rest at the earlest is 1 glycerin 2 water 3 kerosene 4 all of them at the same time

Physical Chemistry
GeneralIncorrect statement about PCI5 molecule is Central atom is sp d hybridised The shape of the molecule is trigonal bipyramidal Axial bonds are shorter than equatorial bonds Three P Cl bond lie in one plane

Physical Chemistry
EquilibriumAt 525 K PC15 g is 80 dissociated at a pressure of 1 atm Now sufficient quantity of an inert gas at constant pressure is introduced into the above reaction mixture to produce inert gas partial pressure of 0 9 atm What is the percentage dissociation of PC1 g when equilibrium is re established a 97 3 c 65 6 b 80 d 4 7

Physical Chemistry
Solutionsd 60 23 38 Insulin C2H1005 n is dissolved in a suitable solvent and the osmotic pressure T of solutions of various concentrations C g cm is measured at 20 C The slope of a plot of against C is found to be 4 65 x10 3 The molar mass of the insulin is 3 a 4 8x105 b 9 105 d 5 17 106 c 3x105

Physical Chemistry
SolutionsWhich of the following azeotropic solutions ha the boiling point less than the boiling point of its constituents molecules 1 CHCl3 and CH3COCH 3 CH3COOH C6H5NH 2 3 4 All of these CH3CH OH and CH3COCH3

Physical Chemistry
Solutions3 Wolframite is FeWO MnWO 4 Cassiterite and rutile are sulphides ore The osmotic pressure of solution containing 34 2 g of cane sugar molar mass 342 g mol in IL of solution at 20 cis Given R 0 082 L atm K mol 1 2 40 atm 2 3 6 atm 3 24 atm 4 0 0024 atm C H OH CHCI NaOH Salicylaldehyde the electrophile involved in the above reaction is

Physical Chemistry
Chemical kineticsA first order reaction is found to have a rate constant k 5 5 x 104 s Example 47 Find the half life of the reaction Half life for a first order reaction is Solution ty a T k 0 693 k 0 693 5 5x10 14s ty 2 T 1 26 x 10 s Show that in a first order reaction time required for completion of 99 9 is 10 times of half life t 2 of the reaction When reaction is completed 99 9 R R 0 999 R 2 303 R o log R t 2 303 t R o R 0 999 R log t 6 909 k For half life of the reaction t1 2 0 693 k 6 909 k 10 2 303 t log10 Example 4 8 Solution

Physical Chemistry
Generalc 100 d zero The molal boiling point constant of water is 0 53 When 2 mole of glucose are dissolved in 4000 g of wa the solution will boil at a 100 53 C 100 265 C b 101 06 C d 99 47 C

Physical Chemistry
Atomic Structures BOOKLET 1 In a mixture of sample of H atoms and He ions electrons in all the H atoms and He ions are present in n 4th state Then find maximum number of different spectral lines obtained when all the electrons make transition from n 4 upto ground state 4 1 32423

Physical Chemistry
Gaseous and liquid statesA real gas becomes ideal at Low temperature and high pressure Low temperature and low pressure High temperature and high pressure High temperature and low pressure

Physical Chemistry
Chemical kineticsA catalyst lowers the activation energy of a reaction in such a manner that the rate constant at 27 C for uncatalysed reaction equals the rate constant at 73 C for catalysed reaction By how many kJ mole activation energy barrier is reduced by catalyst Activation energy for the uncatalyzed reaction is 24 kJ

Physical Chemistry
General12 If law of conservation of mass was to hold true then 20 8 g of BaCl on reaction with 9 8 g of H SO will produce 7 3 g of HCl and BaSO equal to a 11 65 g b 23 3 g c 25 5 g d 30 6 g

Physical Chemistry
SolutionsIn the process shown in the figure on an ideal diatomic gas the value of q and AH respectively is PA A79 5 P V and 94 5 P V C 12 P V and 0 the reaction COCI n 7P P V 4V B 55 5 P V and 94 5 P V D 79 5 P V and defined P varies

Physical Chemistry
General8 6 g of carbon combines with 32 g of sulphur to form CS 12 g of C also combine with 32 g oxygen to form CO 10 g of sulphur combines with 10 g of oxygen to form Sulphur dioxide Which law is illustrated by this a Law of multiple proportions b Law of constant composition c Law of reciprocal proportions d Gay Lussac s law

Physical Chemistry
Generalin a reaction N2 3H2 gives 2 NH3 40 ml of each N2 and H2 are taken to reac t together so that 25 in the of NH3 wa s obtained the volume of N2 present in the container is a 13 33ml b 36 67ml c 26 67ml

Physical Chemistry
Gaseous and liquid statessystem yielded a steady state error of 0 20 for unit step input A unit integrator is cascaded to this system and unit ramp input is applied to this modified system What is the value of steady state error for this modified system a 0 10 c 0 20 b 0 15 d 0 25 ten perm

Physical Chemistry
GeneralWhich of the following samples contains the largest number of atoms C 1 g of N g A 1 g of Ni s B 1 g of Ca s Which of the following contains greatest number of oxygen atoms A 1 g of O B 1 g of 0 2 all have the same number of atoms D 1 g of B s

Physical Chemistry
Nuclear chemistryWhat particle is needed to complete the following equation 56 Mn 25 O O O 56 27 Co 56 Fe 26 27 25 Mn 58 20 Cr D

Physical Chemistry
Gaseous and liquid statesFor a fixed mass of a gas consider the following graph for an ideal gas P P2 P4 P3 P P T C Correct relation between P P2 P3 and P4 is P P P3 P4 P P2 P4 P3 P P4 P3 P1 P2

Physical Chemistry
ElectrochemistryThe reduction potential for all other half reactions are measured relative to the half reaction 2H aq 2e H g Under standard conditions pH 0 000 PH2 1 atm this standard hydrogen electrode SHE is defined to have a reduction potential of 0 000 V 2nd attempt See Periodic Table See

Physical Chemistry
GeneralC H OH CHCI 1 Dichloromethyl 2 Dichlorocarbene CCL 3 Trichloromethyl anion CCI 4 Formyl cation CHO NaOH Salicylaldehyde the electrophile involved in the above reaction cation CHCI

Physical Chemistry
SolutionsThe boiling point of acetic acid is 118 1 C and its latent heat of vaporisation is 121 cal gm A solution containing 0 4344 gm anthracene in 44 16 gm acetic acid boils at 118 24 C What is the molecular wt of anthracene 1 122 2 178 3 105 4 154 1

Physical Chemistry
Chemical Bonding1 1 1 1 2 1 2 1 3 1 2 3 4 2 2 3 3 From a measurement of the freezing point depression of benzene the molecular weight of acetic acid in a benzene solution was determined to be 100 The percentage association of acetic acid is 1 79 2 93 3 80 An aqueous solution containing an 4 100 ionic salt having

Physical Chemistry
General11 7 g of impure sodium chloride is treated with excess of AgNO3 solution 14 35 g of silver chloride M 143 5 g mol precipitate is formed What is the percentage purity of sodium chloride A B C 40 50 60 20

Physical Chemistry
Gaseous and liquid statesIn the van der Waals gas equation the term which indicates the volume occupied by the gas molecules is V nb an V nb P hr min an V

Physical Chemistry
Gaseous and liquid statesConsider the following statements a For ideal gas Z 1 at all temperature and pressure b 0 is more compressible than H c The temperature at which a real gas obeys ideal gas law over an appreciable range of pressure is called critical temperature The correct statements are a and b only b and c only a and c only a b and c

Physical Chemistry
Solutions4 None of these 1 1 44 2 56 10 equivalent of KOH is required to neutralize 0 12544g H XO The atomic mass of X ing mol is Given H XO is a dibasic acid 2 8 1 16 3 7 Which one of the following compounds is a peroxide 2 BaO 1 KO 3 MnO 4 32 4 NO

Physical Chemistry
Chemical kineticsEXAMPLE 2 14 A solution containing 0 1 mol of naphthalene and 0 9 mol of benzene is cooled out until some benzene freezes out The solution is then decanted off from the solid and warmed upto 353 K where its vapour pressure was found to be 670 mm The freezing point and boiling point of benzene are 278 5 K and 353 K respectively and its enthalpy of fusion is 10 67 kJ mol Calculate the temperature to which the solution was cooled originally and the amount of benzene that must have frozen out Assume ideal behaviour

Physical Chemistry
Chemical Bondingsertion When a mL of a 0 1 molal urea solution is mixed with another b mL of 0 1 molal glucose solution the boiling point of the solution is no different from the boiling points of the samples prior to mixing but if a mL of 0 1 molal urea is mixed with b mL of 0 1 molal HF the boiling point of the mixture is different from the boiling points of the separate samples eason HF is an electrolyte weak whereas glucose is a non electrolyte 1 If both the assertion and the reason are true and the reason is a correct explanation of the assertion 2 If both the assertion and reason are true but the reason is not a correct explanation of the assertion 3 If the assertion is true but the reason is false 4 If both the assertion and reason are false 675

Physical Chemistry
GeneralIn ground state of Cu The no of shells occupied subshells filled orbitals and unpaired electrons respectively are P A 4 8 15 0 B 3 6 15 1 en fat anter zuchter HRT Thach Bik

Physical Chemistry
Chemical BondingIn the parallel reaction A 4B 5C if at any time B C 1 and half life period for the formation of B is 5 400 minutes and half life period for the formation of C in nearest possible integers is 5X minutes then X is
Physical Chemistry
ElectrochemistryWhen same quantity of electricity is passed for half an hour the amount of Cu and Cr deposited are respectively 0 375g and 0 30g Ratio of electro chemical equivalent of Cu and Cris B n 0 8 1 25 2 5 1 62

Physical Chemistry
GeneralConsider the following statements a o nitrophenol forms intramolecular hydrogen bond b Boiling point of H S is greater than H O c Hydrogen bond is stronger than covalent bond The incorrect statement s is are a only a and c only a and b only b and c only

Physical Chemistry
GeneralConsider the following statements a Greater is the viscosity more slowly the liquid flows b Glass is an extremely viscous liquid c Sl unit of viscosity coefficient is N m The incorrect statement s is are b only c only b and c only a and c only

Physical Chemistry
SolutionsThe total vapour pressure of a 8 mole solution of NH3 in water at 293 K is 100 0 torr the vapour pressure of pure water is 34 0 torr at this temperature Applying Henry s and Raoult s laws calculate the total vapour pressure for a 10 mole solution A 58 25

Physical Chemistry
General1g Mg was burnt in a closed vessel containing 2g oxygen Which of the following statement is correct 1 0 25g of Mg will be left unburnt 2 0 33gofoxygen will be left unreacted 3 2 5g of MgO will be formed mix will weigh 3g

Physical Chemistry
Solutions2 The ratio of freezing point depression values of 0 01 M solutions of urea common salt and Na SO4 are 1 1 1 1 2 1 2 1 3 1 2 3 4 2 2 3 3 From a measurement of the freezing point depression of