Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
General2CO g CO g C s This question asks to calculate the equilibrium k of the reaction at 800 C and 2 bar in additi to the degree of advancement of the reaction in equilibrium and the partial pressures of CO at CO2 in equilibrium

Physical Chemistry
EquilibriumGiven the following equilibrium reaction identify the effect each change will have as the reaction shifts to reestablish equilibrium CO g C s O g AH 256 kJ Decide if each change would cause the reaction to shift to favor reactants products or cause no change Change Increase in temperature Increase in concentration of C s Decrease in O g Add a catalyst Increase in concentration reactants Effect on Equilibrium

Physical Chemistry
Energetics3 Assuming that the dry matter of the wood biomass consists of 42 of cellulose calculate the efficiency of the SSF Simultaneous Saccharification Fermentation Compare with the efficiency of the SSF reaction performed on starch Explain C6H12O6 2 C2H5OH 2 CO2

Physical Chemistry
Gaseous and liquid statesA sample contains two different ionic species at different concentrations The two ions can be distinguished in polarography by O Half wave potentials Polarography is confined to O solutions containing single type of ions only By addition of one ion such that their concentrations are equal O Diffusion currents

Physical Chemistry
EquilibriumSolid NH HS dissociates into NH3 and H S at a certain temperature the equilibrium pressure is P atm If now NH3 is pumped into the system so that its partial pressure becomes P atm then what will be the partial pressure in atm of H S b 0 25 P d 0 67 P a 0 5 P c 0 33 P

Physical Chemistry
EnergeticsCalculate AG and w for an isothermal expansion of 1 00 mol of an ideal gas wherein the external pressure changes as a function of internal pressure Pex 2P 100 Vatm from 2 478 L to 24 78 Lat 298 K

Physical Chemistry
Generalbases are sufficiently strong to deprotonate a termin alkyne pka Select all apply Points will be deducted for incorrect answers NaH pKa of H 35 NaOCH3 pK of CH3OH 16 LIOH PK of H O 15 7 H O pK of H3O 1 7 CH3Li pK of CH4 60 KOC CH3 3 PK of CH3 3COH 18 n BuLi pK of CH3CH CH CH3 50 NaNH pK of Hy 36 3

Physical Chemistry
EnergeticsConsider the reaction 6CO g 6H O 1 C6H12O6 60 g for which AH 2 801x10 kJ and AS 259 0 J K at 298 15 K 1 Calculate the entropy change of the UNIVERSE when 2 260 moles of CO g react under standard conditions at 298 15 K ASuniverse J K 2 Is this reaction reactant or product favored under standard conditions 3 If the reaction is product favored is it enthalpy favored entropy favored or favored by both enthalpy and entropy If the reaction is reactant favored choose reactant

Physical Chemistry
General1 386 g of p aminophenol MW 109 13 g mol is reacted with 2 926 g of acetic anhydride MW102 09 g mol to form acetaminophen 151 16 g mol and acetic acid 60 052 g mol as shown in the reaction below What is the theoretical yield of acetaminophen CH3 suzu CH3 HO HC A 0 7627 g 1 610 g 3 126 9 1 920 g CH p aminophenol NH H C acetic anhydride HO HC NH CH HO acetaminophen acetic acid CH3

Physical Chemistry
General2 Metal bicarbonates on heating get decomposed to form some basic compounds and gas X Cold and concentrated solution of sodium chloride reacts with ammonia and gas X to produce an acidic salt Y and a basic salt Z Which of the following is not true about Z 0 8 1 It is sparingly soluble in water 2 It has two water molecules as water of crystallisation 3 It is used in soda acid fire extinguishers 4 It releases gas X on heating 4

Physical Chemistry
Equilibrium67 At 27 C and 1 atm pressure N O4 is 20 dissociation into NO What is the density of of N O4 and NO2 at 27 C and 1 atm equilibrium mixture a 3 11 g litre b 2 11 g litre c 4 5 g litre 600 ding th ation COCI Lo d None of these COLCC When heater
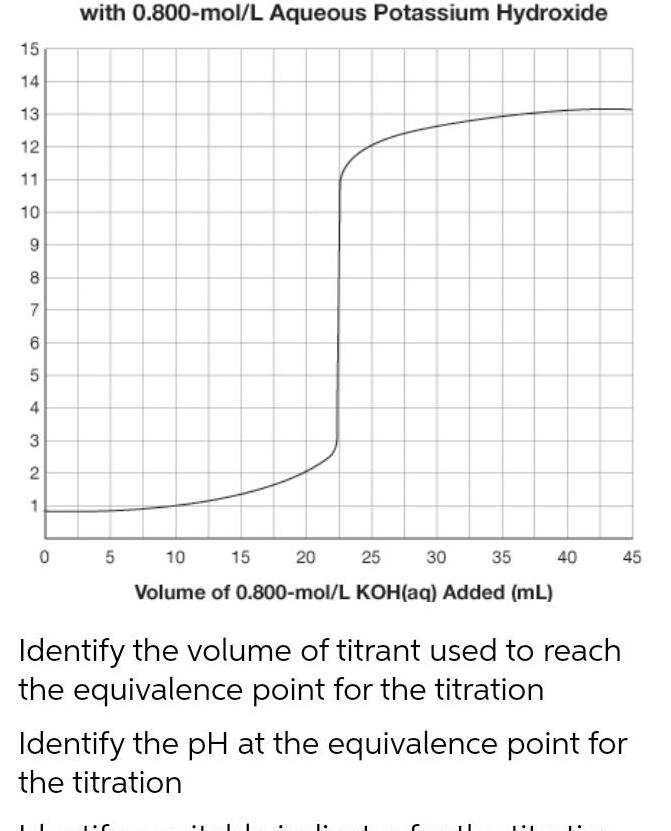
Physical Chemistry
General15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 with 0 800 mol L Aqueous Potassium Hydroxide 5 10 15 20 30 35 40 25 Volume of 0 800 mol L KOH aq Added mL Identify the volume of titrant used to reach the equivalence point for the titration 45 Identify the pH at the equivalence point for the titration

Physical Chemistry
Solutions12 Two pure liquids A and B have vapour pressures in the ratio 1 3 The mol fraction of A in vapou phase when A and B are mixed in 1 3 molar rati in a solution will be 1 0 1 3 0 3 2 0 2 4 0 5

Physical Chemistry
GeneralFor the reaction H g CO g CO g H O g if the initial concentration of H CO and moles litre of hydrogen is consumed at equilibrium the correct expression of Kp x is a 2 x b 1 x 1 x c 1 x d 2 x 2 1 x

Physical Chemistry
Equilibrium10 13 Describe how you would prepare exactly 100 mL of 0 100 M picolinate buffer pH 5 50 Possible starting materials are pure picolinic acid pyridine 2 carboxylic acid FM 123 11 1 0 M HCI and 1 0 M NaOH Approximately how many milliliters of the HCI or NaOH will be required

Physical Chemistry
GeneralThere is a 190 kg sample in the natural state It dries in the oven and its weight is noted 186kg The absorption test is carried out and its SSD weight equal to 194kg is determined The specific weight of the material is equal to 2350 kg m and its unit weight is 1750 kg m Decide Moisture content absorption water total water free water absolute volume apparent volume void volume and of sample voids

Physical Chemistry
Atomic StructureIf n and I are respectively the principle and azimuthal quantum numbers then expression fo calculating total number of electrons in any energ level is 1 n 1 2 21 1 I 1 I n 1 3 2 21 1 1 0 I n 1 2 2 21 1 I 1 I n 1 4 2 21 1 1 0 total number of electron in an energy level orbit nikalne ka formula toh 2n hota hai so why in all options they have given formula of no of electrons in a sub energy level

Physical Chemistry
Equilibriumthe equilibrium constant for the reaction 2SO g O g 2SO g has a value of 278 at a particular temperature What is the value of the equilibirum constant for the following reaction at the same temperature SO g SO g 1 1 8 10 3 10 O g AIPMT Mains 2012 276 2 3 6 10 3 4 1 3 x 10 5

Physical Chemistry
GeneralQuestion 2 19 marks Answer the questions below that relate to the structure and reactivity between the following species Br OH NH a CIRCLE the most nucleophilic site of all the sites in the neutral structures shown above

Physical Chemistry
Solid stateWhich of the following is not a problem of ion selective electrodes O Can measure only positive ions Output is influences by ionic strength O O O Interference with other ions O Drift in electrode potential during a sequence of measurements

Physical Chemistry
Surface chemistrySupply the missing data in the table below Also determine the FREUNDLICH isotherm constants for the following adsorption test data The liquid volume used in the batch adsorption tests was 1 Liter Mass of Adsorbent Original Concentration of Adsorbate grams in Solution mg L 0 3 45 0 001 0 010 0 100 0 500 3 45 3 45 3 45 3 45 Equilibrium Concentration of Adsorbate in Solution mg L 3 45 3 20 2 81 1 76 1 25 Weight of substance adsorbed mg A B C D E

Physical Chemistry
General6 In case the membrane potential of mitochondrion is 150 mV pH inside a matrix is 7 0 while in intermembrane space 6 5 find how much protons mol can be transferred at standard biological conditions T 310 K pH 7 0 if 5 mol NADH is oxidized with the molecular oxygen

Physical Chemistry
Solutions22 In the following graph vapour against mole fraction of the components Which of the following statement is correct A vapour pressure C X 1 E 1 F H XB Mole fraction of B B D X 1 1 AD represents variation of vapour pressure liquid A with XA 2 BC represents variation of vapour pressure liquid B with XB 3 GH FH EH

Physical Chemistry
GeneralWhen n hexene is heated with Mo203 a t 500 C and 20 atmospheres the follow ing changes takes place A Hybridization of carbon changes B No of C C bond increases C No of C H bond decreases D Percentage of C increases a A B only b C D only c A B C onl y d A B C D

Physical Chemistry
EnergeticsList 1 Condition for the system parameter when Non PV work is zero P dH 0 Tds dq R dU 0 List 11 Comment on the nature of spontaneity o the process 1 Spontaneous 2 Non Spontaneous 3 Equilibrium

Physical Chemistry
General1ST YEAR ISHIKA JAIN JANHAVI SINGH YASHSWANI MISHRA MANAN AGRAWAL KAUSHIKI GUPTA PRASHU SHUKLA VARTIKA CHAUHAN NIKARIKA CHAUHAN NISHTHA BHATNAGAR ISHA PARIHAR ISHITA SINGH RUDRANI DUTT MEHAK SHARMA SAKSHI SHUBHRA YADAV KRITIKA TIWARI SHYAMA PODDAR SWAPNIL RAI YASHIKA TOMAR PRANJAL PANDEY NANDINI GOEL AKSHITA SHARMA Official Literary Society CSE ESE IT CSE IT CSE CSE CSE CS CSE EE CSE CSE ECE CS CS EE CSE CS CSE CSE CS RECRUITMENTS 2022 MMDMETMDMTEMMEDME E EMME 2ND YEAR CSE ASTITV GUPTA KANCHI IT CSE GOPAL GUPTA ANADEE CSE IT KSHITIJ YADAV MANASWINI CSE PRATYUSH TRIPATHI IT SUYASH RASTOGI ECE HARSHIT SHUKLA CSE ABHISHEK TIWARI ECE


Physical Chemistry
GeneralIdentily comply bromine dissolved in carbon tetrachloride 1 It is a solid liquid homogeneous mixtu re 2 After separation bromine cannot be br oken down into simpler substances 3 Carbon tetrachloride has the propertie s of carbon and chlorine 4 Bromine and carbon tetrachloride can be separated by a separating funnel Can you please explain the answer to this q

Physical Chemistry
EquilibriumSolid calcium fluoride and solid barium fluoride are in equilibrium with a solution containing 9 76 103 M barium acetate Calculate the concentration of calcium ion present in this solution calcium M

Physical Chemistry
EquilibriumThe Haber Bosch process involves the combination of nitrogen and hydrogen at high temperatures in the presence of a catalyst to produce ammonia It is currently the main industrial source of ammonia N g 3H g 2NH3 g Assuming that a reaction vessel originally contains 0 350 moles of N and 0 550 moles of H answer each of the following questions a How many moles of NH3 could be produced from these quantities of N and H NH3 0 3667 b After the reaction is complete how many moles of N would remain N 0 1833 c After the reaction is complete how many moles of H would remain H 0 mol mol moles

Physical Chemistry
Gaseous and liquid statesFor the estimation of nitrogen 1 4 g of an organic compound was digested by Kjeldahl method and the M evolved ammonia was absorbed in 60 mL of sulphuric acid The unreacted acid required 20 mL of 10 M 10 sodium hydroxide for complete neutralizaton The percentage of nitrogen in the compound is JEE Main online 2014 A 3 B 5 C 6 D 10

Physical Chemistry
GeneralName the product of chemical reaction of but 1 3 diene with chlorine 1 2 dichlorobutane 1 3 dichlorobut 1 ene 1 4 dichlorobutane O 1 4 dichlorobut 2 ene 1 2 dichlorobut 2 ene


Physical Chemistry
GeneralConsider the following chemical equation 2NaOH H SO4 Na SO 2H O The informations conveyed by this equation are 1 NaOH reacts with H SO to produce Na SO and water 2 For every one molecule of H SO4 two molecules of NaOH are required III Acids and bases are non ionic in nature IV This is not a redox reaction The correct statements are a I and II b II and III

Physical Chemistry
EquilibriumConsider the following physical transformation X s X aq If one were to write an equilibrium constant K for the process Assi K X aq X s K X aq K

Physical Chemistry
GeneralDescribe the position of electrons around one O atom in the Lewis structure of O Select one O a 3 shared electron pairs Ob 3 shared electron pairs 1 lone pair O c 2 shared electron pairs 2 lone pairs O d 1 shared electron pair 3 lone pairs O e 2 shared electron pairs 1 lone pair 4

Physical Chemistry
Nuclear chemistry4 How much energy must be supplied to break a single sodium 23 nucleus into separated protons and neutrons if the nucleus has a mass of 22 983733 amu How much energy is required in BTU mole of this nucleus 15

Physical Chemistry
ElectrochemistryIn an electrolytic cell containing molten Cal2 Select one a Ca is deposited at the anode b Current flows from the cathode to the anode through the solution c Ca2 gains electrons d lodine is released as I e 12 is released at the cathode

Physical Chemistry
SolutionsN 24 What is the weight of available oxygen from a solution of H O if 20 ml of this solution needs 25 ml KMnO for complete oxidation 1 59 30 01 g 2 4 0 25 g 0 5 g

Physical Chemistry
ElectrochemistryThe cell reaction for the Zn H cell is Zn s 2H aq Zn aq H g Standard cell potential is 0 76 V and the cell potential at 298K is 0 45 V Calculate the pH of the cell if Zn 1 0 M and PH 1 0 atm at equilibrium H

Physical Chemistry
Generalonsider the following statements 1 137 a Velocity of electron is th velocity of light b First line of Balmer series will be more intense compared to second line c Radius of third orbit of Li2 ion is 1 5 times more than radius of second orbit of He ion Correct among the following is are 1 a only 2 b c only 3 a c only 4 a b c are correct

Physical Chemistry
EnergeticsWhat is the a molar enthalpy of the ammonium chloride water solution cal mol and b partial molar enthalpy of each component cal g given that mass of NH4Cl 0 103g mass of H2O 100g Specific enthalpy of solution 54 8533 cal g

Physical Chemistry
Atomic StructureIncorrect among the following is are a Number of electrons having m 0 in Na are 7 b Energy of 4s orbital is more than 3d in H atom c Energy of 2s orbital in O is more than carbon 1 a only 2 b only 3 a b only 4 c only

Physical Chemistry
Chemical Bondinge 04 Calculate Bond Pairs BP s 1 mark Calculate Lone Pairs LP s 1 mark Draw Lewis Structure 1 mark VSEPR Notation 0 5 mark Predict of then molecular Shape 0 5 mark Polarity 0 5 mark Provide the reason and justify your answer for the polarity

Physical Chemistry
GeneralPrepare a control chart and evaluate whether or not the data meet each criterion for stability in control chart Sample Recovery Sample Recovery Sample Recovery 1 94 6 10 104 6 18 104 6 2 93 1 11 123 8 19 91 5 3 100 0 12 93 8 83 1 4 122 3 13 80 0 100 8 5 120 8 14 99 2 123 1 6 93 1 15 101 5 96 2 7 117 7 16 74 6 96 9 8 96 2 17 108 5 102 3 73 8 9 20 21 22 23 24 25

Physical Chemistry
Electrochemistryelectrochemical cell Mg s Mg OH 2 s NaOH aq MgCl aq Mg s given that E for the reduction half cell reaction is 2 369 V and E for the oxidation side is 2 690 V at 298 15 K You can assume that the solutions are ideal Enter in your answer to 2 decimal places in units of x10 11 Your Answer E E Answer RT InQ X b b 4ac 2a Constants and Conversion Factors ax bx c 0 fxdx Data for H O Standard values all at 298 15 K PH O 3175 Pa at 298 15K AH H O 285 83 kJ mol T273 15 K 1 1 Arus H 6 008 kJ mol K 0 51 K kg mol Tap 373 15 K dr X R 8 314 J K mol 0 08206 L atm mol K 0 08314 L bar mol K N 6 022 x 1023 mol g 9 81 m s 1 Pa 1 kg m s 1 L 1 dm10 m 1 kJ 1000 J 1 atm 101325 Pa 1 01325 bar 760 Torr 1 atm 1 bar 10 Pa 100 kPa 1 L atm 101 325 J Inx e de dx F 96485 C mol 1J 1 kg m s Avap H 40 646 kJ mol K 1 86 K kg mol M 18 02 g mol

Physical Chemistry
GeneralA student at GCC made a saturated solution of Ca 103 2 by adding 256 gr of Ca 103 in 2 0 L of pure water Calculate the molar solubility of this salt at 298 K Ksp of Ca 103 2 2 0 x 10 6 O 3 9 x 10 3 O 7 9 x 10 3 O 4 4 x 10 3 O We can t tell since too much salt is in water 6 6x10 3

Physical Chemistry
SolutionsA mixture of liquids A and B exhibits ideal behavior At 74 C the total vapor pressure of a solution containing 1 6 moles of A and 2 7 moles of B is 321 mmHg Upon the addition of another mole of B to the solution the vapor pressure increases to 338 mmHg Calculate the vapor pressures of pure A and B at 74 C

Physical Chemistry
EquilibriumExample 7 8 Phosphorus pentachloride decomposes according to the equation PCI g PCl3 g Cl g 1 00 mol of PCl is placed in a closed container of 5 0 L capacity and heated to 523 K What will be the composition of the mixture when equilibrium is attained at 523 K The equilibrium constant K for the reaction at 523 K is 1 80

Physical Chemistry
ElectrochemistryBrass an alloy of copper and zinc can be produced by simultaneously electroplating the two metals from a solution containing their 2 ions A 32 83 A current is applied to the brass If 78 82 of the total current is used to plate copper while the rest goes to plating zinc what is the mass percent of copper in the brass Record your answer with one decimal