Gaseous and liquid states Questions and Answers

Physical Chemistry
Gaseous and liquid statesA certain mass of an ideal diatomic gas contained in a closed vessel is heated i t is observed that the temperature rema ins constant However half the amount of gas gets dissociated the ratio of the heat supplied to the gas initial internal energy of the gas will be

Physical Chemistry
Gaseous and liquid states58 Assertion bcc and hcp has same packing efficiency Reason Both have same number of atoms per unit cell and same arrangement a If both assertion and reason are true and reason is the correct explanation of assertion b If both assertion and reason are true but reason is not the correct explanation of assertion If assertion is true but reason is false c d If both assertion and reason are false AIMS

Physical Chemistry
Gaseous and liquid statesEquation for Boyle s Law is A skipped B C D dP P dP P P dv dv 29 d v dT

Physical Chemistry
Gaseous and liquid statesAt identical temperature and pressure the rate of diffusion of hydrogen gas is 3 3 times that of a hydrocarbon having molecular formula CnH2n 2 The value of n is A 1 B

Physical Chemistry
Gaseous and liquid statesAt 1 0 atm the air in a pilot s lungs occupies 1 0 L The pilot ascends to an altitude where the pressure is 0 50 atm If the pilot holds her breath during her ascent what is the new volume of the air in the pilot s lungs 2 0 L O 0 7L 5 7 L 0 85 L 0 18 L

Physical Chemistry
Gaseous and liquid statesQ 38 The E values of the following reduction reactions are given 3 Fe aq e Fe aq E 0 771V Fe aq 2e Fe s E 0 447V What will be the free energy change for the reaction given below 3 Fe aq 3e Fe s 1 F 96485 Cmol 18 51 kJ mol 1 11 87 kJ mol 1 8 10 kJ mol 1 1041 kl mol 1

Physical Chemistry
Gaseous and liquid statesQ 43 Under similar conditions of pressure and temperature 40 ml of slightly moist HCI gas is mixed with 20 ml of NH3 gas the final volume of the gas at the same temperature and Pressure will be 100 ml 20 ml 40 ml 40ml

Physical Chemistry
Gaseous and liquid statesEx 12 One gram of an alloy of Al and Mg when treated with excess of dilute HCl forms MgCl AlCl3 and hydrogen The evolved hydrogen collected over Hg at 0 C has a volume of 1 20 litres at 0 92 atm pressure Calculate the composition of the alloy Al 27 Mg 24

Physical Chemistry
Gaseous and liquid states1 FeCl KI 2 FeCl SnCl 3 FeCl SnCl 4 HgCl SnCl 64 A fixed mass m of a gas is subjected to transformation of states from K to L to M to N and back to K as shown in the figure Pressure Volume The succeeding operations that enable this transformation of states are 1 Heating cooling heating cooling 3 Heating cooling cooling heating ww 2 Cooling heating cooling heating 4 Cooling heating heating cooling

Physical Chemistry
Gaseous and liquid statesFor a semicrystalline polymer if the change in the enthalpy of fusion is 8 7 kJ mol and the change in the entropy of fusion is 25 1 J K mol The melting temperature of an infinite crystal of this polymer in C is O A 121 B 74 O C 16 D 56

Physical Chemistry
Gaseous and liquid states3 CH CH CN 81 Identify the correct labels of A B and C in the following graph from the option given below Root mean square speed V most probable speed V average speed V no of R molecules speed 1 A VB VC V 3 A VB VC V 2 A VB VC V 4 A VB VC V

Physical Chemistry
Gaseous and liquid statesView In Three elements A B and C having oxidation number 1 3 and 2 respectively which of the following is correct molecular formula 2 3 4 rect Answer 3 A2BC AB 2C3 AB 2C4 A2B3C4

Physical Chemistry
Gaseous and liquid statesAqueous solution of nickel sulphate on treating with pyridine and then adding a solution of sodium nitrate gives dark blue crystals of a Ni py 4 SO4 c Ni py 4 NO 2 b Ni py 2 NO 2 d Ni py 3 NO 2SO4

Physical Chemistry
Gaseous and liquid states1 2 3 56 The weight of silver at wt 108 displaced by a quantity of electricity which displaces 560 mL of O at STP will be volume of 1 mole of gas at STP is 22 4L 57 1 54 9 g 2 5 4 g 3 10 8 g 4 108 0 g The increasing order of basicity for the following intermediates is from weak to strong

Physical Chemistry
Gaseous and liquid states21 Which of the following metal ion forms unstable Cu in this und complex with CN 1 Ag 1 CU CCN 2 Zn II 3 Cu II 4 Cr 1 22 Which of the following ion does not exist 1 Cul 2 VO 3 WO 2 4 CrO 2

Physical Chemistry
Gaseous and liquid statesThe pk value of ammonium hydroxide is 4 75 An aqueous solution of ammonium hydroxide is titrated with HCI The pH of the solution at the point where half of the ammonium hydroxide has been neutralized will be A 9 25 B 8 25 C 7 50 D 4 75

Physical Chemistry
Gaseous and liquid statesA closed cylinder contains 320 g of O gas and 20 g of He gas If partial pressure of O in the cylinder is 5 atm then the total pressure of the gaseous mixture will be O 12 atm O 9 5 atm O 10 atm O 7 5 atm

Physical Chemistry
Gaseous and liquid statesA closed cylinder contains 320 g of O2 gas and 20 g of He gas If partial pressure of O in the cylinder is 5 atm then the total pressure of the gaseous mixture will be 12 atm 9 5 atm 10 atm 7 5 atm

Physical Chemistry
Gaseous and liquid statesQ 10 Water at the rate of 3 8 kg s is heated from 38 to 55 C in a shell and tube heat exchanger On the shell side one pass is used with water as the heating fluid 1 9 kg s entering the exchanger at 93 C The overall heat transfer coefficient is 1419 W m C and the average water velocity in the 1 9 cm diameter tubes is 0 366 m s Because of space limitations the tube length must not be longer than 2 5 m Calculate the number of tube passes the number of tubes per pass and the length of the tubes consistent with this restriction Assume LMTD correction factor F 0 9 for two passes

Physical Chemistry
Gaseous and liquid states64 mL of an unknown gas diffuses in same time as 128 mL of methane gas under identical conditions molar mass of unknown gas is 32 g mol 1 O 48 g mol O 96 g mol hr m O 64 g mol

Physical Chemistry
Gaseous and liquid statesConsider the following statements a At very low temperature intermolecular forces between gaseous molecules become significant b For ideal gas Z 1 at all temperature c At high pressure all gases have Z 1 The correct statements are O a and b only Ob and c only Oa and c only O a b and c

Physical Chemistry
Gaseous and liquid statesConsider the following statements for kinetic molecular theory of gases a Particles of a gas move in all possible directions in straight lines b At any particular time different particles in the gas have different kinetic energies c Collisions of gas molecules are perfectly inelastic in nature The correct statement s is are O a only Ob only a and b only a b and c

Physical Chemistry
Gaseous and liquid statesChoose the correct statement of the following A The specific heat capacity at constant pressure for H g is 7 cal unit amount B The critical volume of a gas is three times of its excluded volume Dalton s Law of partial pressure is applicable to the mixture of H and He at all temperatures D In the diffusion process of a mixture of He and Ne the remaining gas in the container is enriched in

Physical Chemistry
Gaseous and liquid states1 What is the method used in the preparation of the iso alcoholic elixir 2 Compute for the alcoholic strength v v of the prepared low alcoholic elixir 3 What volume in mL of the prepared low and high alcoholic elixir with 55 v v alcoholic strength 4 Why was the low alcoholic elixir allowed to stand for 24 hours 5 Explain why turbidity occurs when an aqueous solution is added to the alcoholic solution 6 A 60 year old male patient was prescribed with an alcoholic elixir with 40 alcohol strength for his cough However he also orders aspirin for his headache What will you tell the patient OmL 1 What is the former official name of the terpin hydrate oral solution 2 What is the method used in the preparation of the terpin hydrate oral solution 3 Compute for the alcoholic strength in v v of the prepared elixir 4 Does the prepared product comply with the terpin hydrate content stated in the USP monograph 5 In the preparation of elixirs talc is most commonly used as filter aid to remove turbidity Explain how this process works 6 Givr the role of the orange peel tincture benzaldehyde glycerin and syrup in the preparation Explain why the adjunctive solvents glycerin and propylene glycol are commonly added in manufacturing elixirs 7 8 Aside from sucrose what other material are used as sweeteners in preparing elixirs

Physical Chemistry
Gaseous and liquid statesAt a certain temperature for which RT 25 lit atm mol the density of a gas in gm lit is d 2 00 P 0 20 P where P is the pressure in atmosphere The molecular weight of the gas in gm mol If your answer is x write the value of x 10 IS

Physical Chemistry
Gaseous and liquid states1 1 I and II II 2 I and III 4 III and IV 57 The temperature of a sample of gas is raised from 127 C to 527 C The average kinetic energy of the gas 1 Does not change 2 is doubled 3 is halved 4 cannot be calculated In the radioactive decay of X which of the following could be considered as incorrect statement 58 III 3 II and IV IV

Physical Chemistry
Gaseous and liquid statesNegative Marks In all other cases An ideal gaseous mixture of ethane C H and ethene C H occupies 28 litre at 300K 1atm The mixture reacts completely with 128 gm O to produce CO and H O Mole fraction at C H in the mixture is Report answer after multiplying with 10 Nearest integer R 0 08 litre atm K mole V Litre of solution A Resistance 50 ohm is mixed with V Litre of solution B

Physical Chemistry
Gaseous and liquid states2 Xenon trioxide XeO3 forms xenate ion in alkaline medium XeO NaOH Na HXCO But the xenate ions slowly disproportionate in alkaline solution as Na HXeO NaOH Z Xe O H O The compound Z is expected to be 1 Na XeO 2 Naz XeO4 3 Na4 XeO6 4 Na4 XeO

Physical Chemistry
Gaseous and liquid statesConsider the equation Z PV nRT which of the following statements is correct a When Z 1 real gases are easier to compress than the ideal gas b When Z 1 real gases get compressed easily c When Z 1 real gases are difficult to compress d When Z 1 real gases are difficult to compress

Physical Chemistry
Gaseous and liquid states0 A 0 60 g sample consisting of only CaC O4 and MgC O4 is heated at 500 C converting the two salts of CaCO3 and MgCO3 The sample then weighs 0 465 g If the sample had been heated to 900 C where the products are CaO and MgO what would the mixtures of oxides have weighed A 0 12 g C 0 252 g B 0 21 g D 0 3 g

Physical Chemistry
Gaseous and liquid statesCrCl 6H O exists in different isomeric forms which show different colours like violet and green This is due to a Ionisation isomerism b Coordination isomerism c Optical isomerism d Hydrate isomerism

Physical Chemistry
Gaseous and liquid statesAn ideal gas can be expanded from an initial state to a certain volume through two different processes A PV2 K B P KV2 where K is a positive constant Then choose the correct option from the following 1 Final temperature in A will be greater than in B 2 Final temperature in B will be greater than in A 3 Work done by the gas in both the processes would be equal 4 Total heat given to the gas in A is greater than in B

Physical Chemistry
Gaseous and liquid statesFor a real gas obeying van der waals equation which of the following is are true A Internal energy of gas is dependent on temperature only B If Z 1 then forces of repulsion are dominant C At very high pressure for most of the gases Z 1 1 RD RT D The second virial coefficient B depends on the nature of gas and temperature

Physical Chemistry
Gaseous and liquid states9 A Mixture of Cyclopropane gas and oxygen is used as an anesthetic cyclopropane containes 85 7 C and 14 3 H by mass At 50 C and 0 984 atm pressure 1 56 g cyclopropane has a volume of 1 00 L what is the molecular formula of cyclopropane

Physical Chemistry
Gaseous and liquid statesSolve the following 1 A sample of argon has a volume of 4 Liters when the pressure is 1 atm What volume doe aragon occupy at 1 5 atm 2 200mL of hydrogen gas is kept at constant temperature it has a pressure of 1 5atm Calculate the pressure when compressed to

Physical Chemistry
Gaseous and liquid states81 AAJ KA TOPPER An unknown compound A dissociates at 500 C to give products as follows 4 g B g C g D g Vapour density of the equilibrium mixture is 50 when it dissociates to the extent to 10 What will be the molecular weight of compound A 1 120 2 130 3 134 4 140 MY CCOON

Physical Chemistry
Gaseous and liquid states34 Select the correct statement a If H y 10x then pH x log y X 1 10 then pH x log y c pH of a solution 14 log OH d All of the above b If H y

Physical Chemistry
Gaseous and liquid statesQuestion No 37 In the van der Waals gas equation the term which indicates the volume occupied by the gas molecules is V nb O O O an v nb an P a

Physical Chemistry
Gaseous and liquid states11 Assertion Most probable velocity is the velocity possessed by maximum fraction of molecules at the same temperature Reason On collision more and more molecules acquire higher velocity at the same temperature

Physical Chemistry
Gaseous and liquid statesQuestion No 36 Pressure exerted by 16 g of oxygen gas in 0 01 m vessel at 47 C is O 4 5 atm O 1 3 atm 0 21 atm 6 8 atm
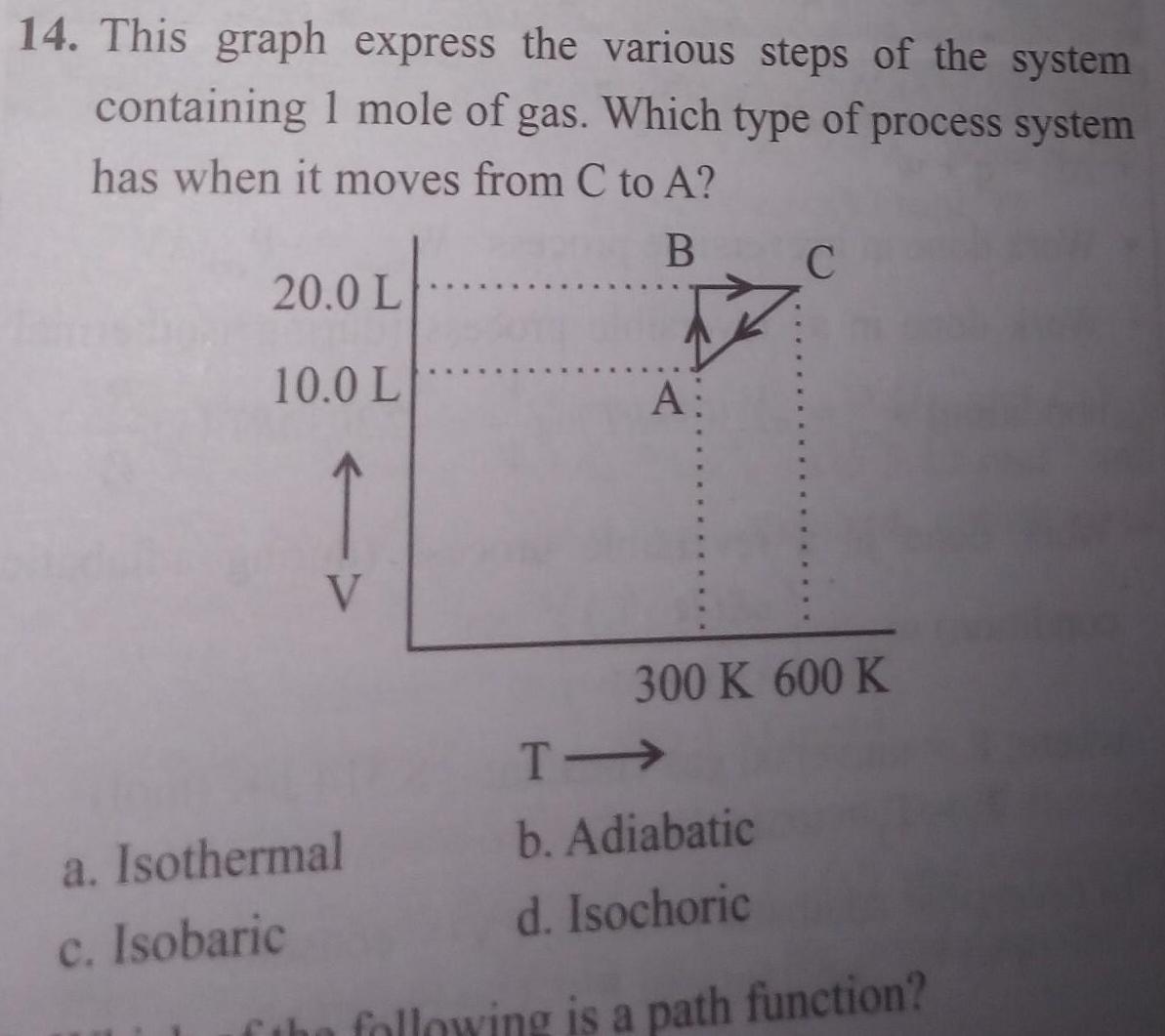
Physical Chemistry
Gaseous and liquid states14 This graph express the various steps of the system containing 1 mole of gas Which type of process system has when it moves from C to A B 20 0 L 10 0 L V A a Isothermal c Isobaric C 300 K 600 K T b Adiabatic d Isochoric the following is a path function

Physical Chemistry
Gaseous and liquid states31 At constant temperature the equilibrium constant K for the decomposition reaction N O g 2NO is expressed by 4 K P 4x P 1 x where P pressure x extent of decomposition Which of the following statements is true a K increases with increase of P P b K increases with increase of x P c K increase with decrease of x P d K remains constant with change in P or Y

Physical Chemistry
Gaseous and liquid states28 The work done on the system when one mole of an ideal gas at 500 K is compressed isothermally and reversibly to 1 10th of its original volume R 2 cal a 500 kCal b 1 51 kCal c 23 03 kCal d 2 303 kCal

Physical Chemistry
Gaseous and liquid statesA gas at 350 K and 15 bar has molar volume 20 percent smaller than that for an ideal gas under the same conditions The correct option about the gas and its compressibility factor Z is NEET 2019 1 Z 1 and attractive forces are dominant 2 Z 1 and repulsive forces are dominant 3 Z 1 and attractive forces are dominant 4 Z 1 and repulsive forces are dominant

Physical Chemistry
Gaseous and liquid statesincorrect 0 00 points out of 10 00 Flag question Consider the following reaction equation 3 H g N g 2 NH3 g K 2 96 10 at a certain temperature At equilibrium the partial pressure of the hydrogen gas is 0 779 atm and the partial pressure of the nitrogen gas is 0 665 atm What is the partial pressure of ammonia in the equilibrium mixture at this temperature Select one 0 00965 atm 9 310 6 atm 0 00392 atm x 0 00346 atm 0 00305 atm

Physical Chemistry
Gaseous and liquid statesQuestion No 41 The ratio of most probable speed of Helium at 100 K to that of O2 at 400 K is O 2 1 O 3 1 O 4 1 O 2 1

Physical Chemistry
Gaseous and liquid states1 Assertion The equation PV nRT is not applicable to real gas Reason For real gases the attractive forces between the molecules cannot be neglected 8

Physical Chemistry
Gaseous and liquid statespartial pressures 14 Assertion 1 4th of the initial mole of the air is expelled if air present in an open vessel is heated from 27 C to 127 C Reason Rate of diffusion of a gas is inversely proportional to the square root of its molecular mass

Physical Chemistry
Gaseous and liquid statesA vessel has 6 g of oxygen at a pressure P and temperature 400 K A small hole is made in it so that O leaks out How much O leaks out if the pressure is and temperature is 300 K P 2 1 5 g 3 2 g 2 4 g 4 3 g

Physical Chemistry
Gaseous and liquid statesThe cylinder contains 100gm of an ideal gas mol wt 40 gm mol at 27 C and 2atm pressure In transportation the cylinder fell and a dent was created The valve present cannot keep the pressure greater than 2atm Hence 10 gm of a gas got leaked out The volume of the container before and after dent is 4 27 7L 27 7L 1 30 8L 27 7L 2 2771 3081 3 30 ST 3081 Which of the following constitutes a set of amphoteric species