Atomic Structure Questions and Answers

Physical Chemistry
Atomic Structure17 Electronic configuration of an atom 1s 2s 2p6 3s 3p6 3d5 4s 1s 6 2p 2s ORG 3s 3p 3d 4s Choose the correct statement regarding this E C 1 It represents the ground state of Cr 2 It violates Aufbau principle 3 It violates Hunds rule of maximum multiplicity

Physical Chemistry
Atomic StructureThe work function for Ag metal is 7 5 x 10 19 J Ag metal is being exposed to the light of frequency 1220 Which is are correct statements 1 Threshold frequency of metal is 1 135 1015 s 1 2 Threshold frequency of metal is 8 33 1015 S 1 3 Stopping potential is 5 46 volt 4 If light of wavelength 3600 is used then phot

Physical Chemistry
Atomic StructureStructure of Atom 231 A light of frequency 1016 Hz is irradiated on a metal surface with threshold frequency equal to 5 1015 Hz What would be the maximum kinetic energy of photoelectrons emitted 1 3 313x10 15 J 2 3 313x10 18 J 3 1 02 1016J 4 4 316x10 18 J

Physical Chemistry
Atomic StructureA hydrogen atom and a He atom are approaching each other with speed v 2v respectively The minimum kinetic energy that He atom must have so that both the atom can excite to 1st excited state will be A 20 4 eV B 113 33 eV C 81 6 eV D 102JeV

Physical Chemistry
Atomic StructureRadius of first excited state of Be ion is NCERT Pg 48 1 13 22 pm 2 52 9 pm 3 105 8 pm 4 211 6 pm Energy required to excite the electron in a hydrogen atom from 2nd to 3rd orbit is NCERT Pg 48

Physical Chemistry
Atomic Structure13 Which of the following set of quantum numbers is not possible NCERT Pg 56 1 1 n 3 1 2 m 2 s 2 1 2 n 4 1 0 m 0 s NI 2 1 3 n 2 1 1 m 0 s 7 2 14 Total number of nodes subshell is 1 4 n 5 1 3 m 2 s 2 present in 3d INCER FOL 16 4 dxz Energy of which hydrogen atom 1 6s 3 4d 17 According to Aufba orbital takes pla multielectron atom 1 5p 3 4f 18 Maximum number

Physical Chemistry
Atomic Structure4 Energy of one mole of photons of radiation whose frequency is 2 x 1014 Hz is nearly 1 80 kJ 3 247 kJ NCERT Pg 43 2 153 kJ 4 366 kJ 2 3 4 h 5T SE 5h 2

Physical Chemistry
Atomic Structure16 Energy of which orbital is maximum for hydrogen atom NCERT Pg 61 1 6s 3 4d 2 5p 4 5f 17 According to Aufbau principle filling of which orbital takes place just after 5s in a multielectron atom NCERT Pq 621 20

Physical Chemistry
Atomic StructureElectrons are filled in orbitals following Aufbau Principle Pauli s Exclusion Principle and Hund s Principle Quantum number are assigned to get complete information of electrons regarding their energy angular momentum etc d a s Gum 340g far dacia af 3 fuqia Aun a gue Meld xgar vi gial Fich Gui tuna H n f qui Gh rt ua B H Which of the following set of orbitals are arranged in correct energy order A 3p 3s 2p in He B 3px 3s 2p in H C 4p 4p 4p in He D 4px 4py 4p 4s 4dxy in H

Physical Chemistry
Atomic Structure41 When copper metal is treated with dilute nitric acid copper II nitrate and nitric oxide forms Find the coefficient of copper II nitrate in the balanced equation of this reaction A 2 B 4 C 1 D 3

Physical Chemistry
Atomic StructureIf according to De Broglie electron moves in a specific orbit like a wave and centripetal f orce only works when a object is moving in circlular path then how is the electron held i n the orbit knowing that according to uncert ainty principle it cannot exist in nucleus Nucleus r I

Physical Chemistry
Atomic StructureWhen electromagnetic radiation of wavelength 310 nm fall on the surface of Sodium electrons are emitted with K E 1 5 eV Determine the work function W of Sodium A 2 5 eV B 5 eV C 10 eV D 12 5 eV

Physical Chemistry
Atomic StructureWhich of the following statements is true The maximum number of electrons can be accommodated in M shell is 8 B The atomic radius increases moving from left to right along a period C The effective nuclear charge increases across a period D The electronegativity of elements decreases from left to right along a period

Physical Chemistry
Atomic StructureIf neutron electron proton and a particle have same momentum mv then which of the following is correct All have same de Broglie wavelength All have same velocity O All have same kinetic energy All have different de Broglie wavelength

Physical Chemistry
Atomic Structureestion No 5 40 If a box containing large number of balls identical in each and every asp which statistics can be applied to balls AO B E statistics BOF D statistics CCO No statistics DO M B statistics 20064696 202 4696 2020064 6 2020064696 020064696

Physical Chemistry
Atomic StructureAn atomic orbital X has 2 radial nodes but onlyone angular node An another atomicorbital Y has oneradial node but two angular nodes The correct information s regarding these orbitals is are C D X is 4p orbital Y is 4d orbital When an electron jumps fromorbital X to orbital Y inH atom energy is absorbed When an electron jumps fromorbital Y to orbital X inHe atom energy is released

Physical Chemistry
Atomic StructureThe approximate size of the nucleus can be calculated by using energy conservation theorem in Rutherford s a scattering experiment i e Total energy initial Total energy final In 1st experiment radius of a particular nucleus is calculated by the projection of a particle from infinity at a speed v m s Let this radius be the true radius In 2nd experiment the radius calculation for the same nuclei is made by projecting the a particle with five sixth 5 6 of the earlier speed The percentage error involved in the calculation of radius of nucleus in the 2nd experiment is

Physical Chemistry
Atomic StructureThe wave function of two different orbitals X and Y in Be 3 ion are given by 1 3 2 Z 6 60 e 0 2 ao 1 3 1 2 y y 9 6 47 2 4 0 e 0 2 2zr nao 4x 1 1 2 9 3 4T 1st 3 2 where ap From this we can conclude cose Bohr radius of H atom z Charge number of nucleus r distance from nucle Options 1 Total number of nodal surface is same for both orbitals TT 2 The angular nodal surface of Y orbital will occur at 0 3 Energy of electron in Y orbital is greater than in X orbital 1 vorggo distance of X orbital from nucleus is greater than Y orbital

Physical Chemistry
Atomic Structure6 Ionization energy of gaseous Na atoms is 495 5 kjmol The lowest possible frequency of light that ionizes a JEE MAINS ONLINE 2014 sodium atom is 3 1 24 x 1015 S 1 4 7 50 x 104 S 1 h 6 626 x 10 34 Js NA 6 022 x 1023 mol 1 3 15 x 10 5 S 1 2 4 76 x 1014 S 1

Physical Chemistry
Atomic StructureSummary of Dual Nature of Radiation The various phenomena concerning radiation can be divided into three parts i The phenomena such as interference diffraction polarisation etc in which there is interaction between radiation and radiation can be explained on the basis of wave nature of radiation The phenomena such as black body radiation photoelectric effect etc in which there is interaction between radiation and matter can be explained on the basis of quantum theory of radiation i e particle nature iii The phenomena such as rectilinear propagation reflection refraction etc in which neither there is interaction between radiation and radiation nor between radiation and matter can be explained with either of the concepts i e either by wave theory or by photon theory 2 x 10 5 6 63 10 19 1 10 5 1 6 63 x 10 34 4 The number of photons of light having waveleng nm which can provide 1 J energy is nearly a 107 photons c 5 x 10 7 photons b 5 x 10 8 photons d 5 x 107 photons Ans c Hint E hc n EX hc 1x 100 x 10 6 626 x 10 34 3 10 5 10 7 5 The atomic transition gives rise to the radiat frequency 10 MHz The change in energy per r atoms taking place would be a 3 99 x 10 6 J c 6 62 x 10 24 J Ans b b 3 99 J d 6 62 x 10 30 J

Physical Chemistry
Atomic StructureWhich of the following statement s is are incorrect Options 1 The electronic configuration of Cr is Ar 3 d5 4 s Atomic Number of Cr 24 The magnetic quantum number may have a negative value 2 3 In Ruthenium atom 20 electrons have a spin of one type and 24 of the opposite type Atomic Number of Ru 44 A The owvidatic regen in HN in

Physical Chemistry
Atomic StructureA H like species is in their excited state A and absorbs a photon of energy 3 868 eV and get excited to a new state B The electron from B on returning to a lower orbit can give a maximum of ten different emissions Some of the radiations have energies greater than it and some equal to 3 868 eV Exactly 2 radiations have energies less than 3 868 eV Determine the orbit numbers of states A and B and also identify the species

Physical Chemistry
Atomic Structure13 The mass of the nucleus is O A greater than the mass of the protons and neutrons that make up the nucleus OB equal to the mass of the protons and neutrons that make up the nucleus OC less than the mass of the protons and neutrons that make up the nucleus OD converted to energy

Physical Chemistry
Atomic Structureth According to Bohr s theory the energy in the n 2 176 10 18 x Z n remove an electron from the 2nd orbit of Li ion orbit is En 0 A 406 A What is the the longest wavelength of light needed to

Physical Chemistry
Atomic StructureIf uncertainty in the measurements of position and velocity of electron are equal then minimum uncertainty in its velocity will be A B skipped C 1 2m 1 h am h 2 m QUES 1 6 Corr MY P S

Physical Chemistry
Atomic Structure17 The frequency of radiation emitted when the electron falls from n 4 to n 1 in a hydrogen atom will be Given ionization energy of H 2 18 10 8 Jatom and h 6 626 10 4 Js a 1 54 x 10 5 S 1 b 1 03 x 10 5 S 1

Physical Chemistry
Atomic StructureSelect the incorrect statement among the following A The minimum energy that an electron in hydrogen atom can have is 13 6 Ev B The energy of an electron in the 2nd excited state of hydrogen atom is 1 51eV C v AE This expression is called Bohr s frequency rule h D The concept of wave number is specially used in spectroscopic analysis

Physical Chemistry
Atomic StructureIS 2 52 The line at 434 nm in the Balmer series of the hydrogen spectrum corresponds to to th a transition of an electron from the n second Bohr orbit The value of n is

Physical Chemistry
Atomic StructureQ7 Calculate the velocity total energy and radius of an electron moving in the 4th orbit of Be Given velocity of an electron V 2 19 x 108 cm s Also mention two limitations of Bohr s atomic model 11 5

Physical Chemistry
Atomic StructureUncertainty in position of a particle of 25 g in space is 10 5m Hence uncertainty in velocity ms is Planck s constant h 6 6 x 10 34 Js A 2 1 x 10 28 skipped B C D 2 1 x 10 34 0 5 x 10 34 5 0 x 10 24 Jy

Physical Chemistry
Atomic Structure5 Sketch the radial wavefunction the probability density and the probability distribution function of a 3d orbital At what radius will the probability distribution function reach its maximum value

Physical Chemistry
Atomic StructureA sample of hydrogen atoms in ground state absorbs light of x nm If the excited sample emits the radiations of six different photon energies but the detector of these emitted radiations has not detected Ans 0100 ultraviolet radiations then the value of x is Given RH 1 96 x10 m

Physical Chemistry
Atomic Structure175 Arrange the orbitals of H atom in the increasing order of their energy 3px 2s 4dxy 3s 4pz 3py 4s A 2s 3s 3px 3py 4s 4p 4dxy B 2s 3s 3px 3py 4s 4p 4dxy C 2s 3s 3px 3py 4s 4pz 4dxy 20 An Ad r

Physical Chemistry
Atomic Structure15 If n l are principal and azimuthal quantum no respectively then the expression for calculating the total no of electron in any energy level is l n 1 2 2l 1 l 0 3 l n 1 2 2l 1 l 0 l n 1 2 2 2l 1 l 1 l n 1 4 2 2l 1 l 0
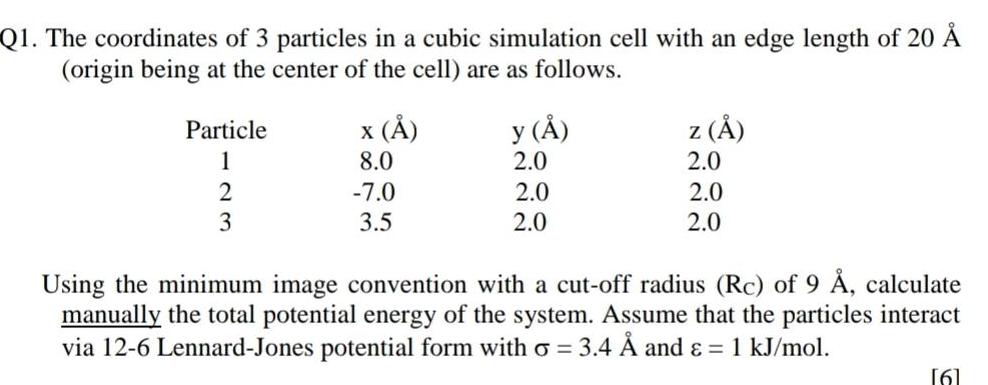
Physical Chemistry
Atomic StructureQ1 The coordinates of 3 particles in a cubic simulation cell with an edge length of 20 origin being at the center of the cell are as follows Particle 1 2 3 x 8 0 7 0 3 5 y 2 0 2 0 2 0 z 2 0 2 0 2 0 Using the minimum image convention with a cut off radius Rc of 9 calculate manually the total potential energy of the system Assume that the particles interact via 12 6 Lennard Jones potential form with o 3 4 and 1 kJ mol 6

Physical Chemistry
Atomic StructureIf an element A shows two cationic states 2 and 3 and form oxides in such a way that ratio of the element showing 2 and 3 state is 1 3 in a compound Formula of the compound will be 1 Ag011 3 A 0 1 2 A 011 4 A5011

Physical Chemistry
Atomic Structurei 6 04 eV Match A Energy of ground state of He B Potential energy of I ii 27 2 eV orbit of H atom C Kinetic energy of II iii 8 72 x10 18 excited state of Het D Ionisation potential iv 54 4 eV of Het 1 A i B ii C iii D iv 2 A iv B iii C ii D i 3 A iv B ii C i D iii 4 A ii B iii C i D iv

Physical Chemistry
Atomic StructureTwo hydrogen atoms collide head on a nd end up with zero kinetic energy Eac h atom emits a photon of wavelength 1 21 6nm Which transition leads to this wavelength a 2 to 1 b 3 to 1 c 4 to 3 d 3 to 2 I looked up in the internet and there the y told that the lower orbit is n 1 since t he wavelength belonged to Lyman serie s But is it necessary that we should re member all the wavelength limits of ea ch series Can t we get both the orbit n

Physical Chemistry
Atomic StructureThe wavelength of first line of the Lyman series for hydrogen is 1216 A The wavelength for the first line of this series for a 10 time ionised sodiun atom z 11 will be 1 1000 A 3 10 A 2 100 A 4 1 A

Physical Chemistry
Atomic StructureWhich of the following set of quantum numbers n l m s represents the unpaired electron of Cu ion Atomic number of Cu 29 1 On 3 1 2 m 2 s 2 1 O n 4 1 0 m 0 s 2 O n 4 1 0 m 0 s On 3 1 1 m 1 s 1 IN 2 1 2

Physical Chemistry
Atomic StructureMarking scheme o 4 If the correct integer is typed in the provided space o O In all other cases An element undergoes a reaction as shown X 2e X2 31 35 eV atom If the energy released is used to dissociate 4 gms of H molecule equally into H H where H is excited state of H atoms where the e travels in an orbit whose circumference is equal to 4 times its de Broglie s wavelength Determine the least no of moles of X that would be required Bond energy of H 5 0 eV molecule

Physical Chemistry
Atomic StructureChemistry Sec 1 Maximum Marks 24 This section has 6 questions The answer to each question is a SINGLE DIGIT INTEGER ranging from 0 to 9 both inclusive Marking scheme o 4 If the correct integer is typed in the provided space o 0 In all other cases Light from a discharge tube containing hydrogen atoms falls on the sodium metal surface The kinetic energy of the fastest moving photoelectron emitted from sodium is 0 73eV If these photons are emitted in hydrogen atom due to the transition from energy 1 to n and the work function of sodium metal is 1 82eV then the minimum value of 1 1 is

Physical Chemistry
Atomic StructureFind number of orbital s in L shell that is are possible when values of magnetic quantum number m 1 to zero to 1 integral value only The total number of compounds shown below that form phenyl hydrozone derivatives under

Physical Chemistry
Atomic Structureo O In all other A single electron orbits around a stationary nucless of charge Ze where Z is a constant and e is the magnitude of electronic charge It requires 47 2 eV to excite the electron from second Bohr orbit to the third Bohr orbit What would be the energy required to excite the electron from n 3 to n 4 in eV

Physical Chemistry
Atomic Structure3 The formula of nickel oxide with metal deficiency defect in its crystal is Nio 98 O The crystal contains Ni and Ni ions The fraction of nickel existing as Ni ions in the crystal is 1 0 96 2 0 04 3 0 50 4 0 31

Physical Chemistry
Atomic StructureIf a photon of energy 14 eV is H atom what is true 1 Atom will be ionised and electron will have a kinetic energy of 14 eV 2 Atom will be ionised and electron will have a kinetic energy of 0 4 eV 3 Photon passes through atom without interacting with it 4 More than one electrons will make transitions

Physical Chemistry
Atomic Structureo 2 In case if selected typed option is incorrect o 0 In case if no response is typed selected Which of the following statement are correct for the transition elements Placed from 3rd to 6th period Last electron enters into n 1 orbital exhibits variable valency General electronic configuration is n 1 d 10 ns0 2

Physical Chemistry
Atomic Structureed option is incorrect o 0 In case if no response is typed selected The energy needed to excite an electron from ground state to the 1st 2nd and 3rd excited states are 10 eV 15eV and 17 eV If 16 eV energy is provided to the electron and if this energy does not match with the energy difference between any two levels the electron O will go to 2nd excited state and 1 eV energy will be converted to its K E O Can go to the 1st excited state and 6eV energy will be converted to K E O will not excite at all O will remain in between 2nd and 3rd excited state

Physical Chemistry
Atomic Structure34 The second order Bragg diffraction of X rays with 1 00 from a set of parallel planes in a metal occurs at an angle 60 The distance between the scattering planes in the crystal is a 2 00 b 1 00 c 0 575 d 1 15 1998 35 The edge length of face centred unit cubic
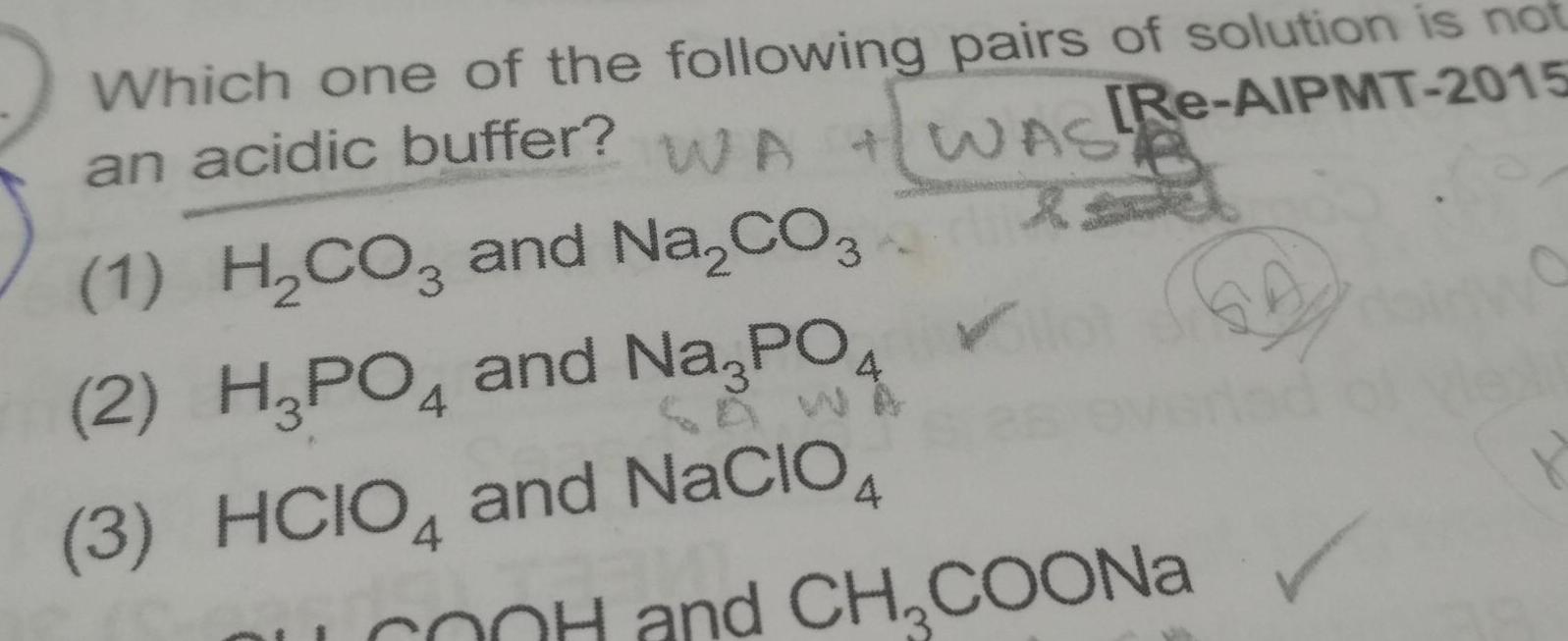
Physical Chemistry
Atomic Structurean acidic buffer WA Which one of the following pairs of solution is not Re AIPMT 2015 WASA 1 H CO3 and Na CO3 2 H3PO4 and Na3PO 3 HCIO4 and NACIO4 4 OOH and CH COONa