Atomic Structure Questions and Answers

Physical Chemistry
Atomic StructureP Oa The state of an ideal gas is changed in a closed path 1 2 3 4 1 Which of the following is true about work done on the gas 1 Work 1 2 W 0 Work 1 2 Ob w 0 Work 1 2 W 0 13 dW 0 4 Work 2 3 W 0 Work 2 3 W 0 Work 1 2 Work 2 3 W 0 V Work 2 3 W 0 Work 3 4 W 0 Work 3 4 W 0 rk 3 W 0 Work 3 4 W 0 Work 4 1 W 0 Work 4 1 W 0 Work 4 1 W 0 Work 4 1 W 0

Physical Chemistry
Atomic Structure2 The wavelength of photon obtained by electron transition between two levels in H atom and singly ionised He are and respectively then 1 2 2 3 2 2 2 4 2 4

Physical Chemistry
Atomic Structure13 10 Suppose you need to know the number of moles of a gas from measurements of its pressure temperature and volume We assume that the gas is ideal a possible source of systematic error Perhaps our measurements of P V and T are P 0 268 0 012 atm V 1 26 0 05 L T 294 2 0 3 K R 0 082058 L atm K mol Report the number of moles of gas with uncertainty Use the calculus based approach

Physical Chemistry
Atomic Structure35 Four lowest energy levels of H atom are shown in the figure The number of emission lines could be 1 3 2 4 3 5 4 6 4 432 95 n 1 In the above problem the number of absorption lines could be t 1 3 2 4 3 5 4 6

Physical Chemistry
Atomic Structure5 What is the correct representation of reaction occurring when HCI is heated with MnO 1 MnO4 5CI 8H 2 MnO 2Cl 4H 3 2MnO 4Cl 8H 4 MnO 4HCI Mn2 5Cl 5H 0 Mn2 Cl 2H O 2Mn 2Cl 4H O MnCl4 Cl H O

Physical Chemistry
Atomic StructureName the substance oxidized and redu ced and also identify the oxidizing agen ts and reducing agents in the following reaction 1 Fe203 3Co 2Fe 3Co2 2 3MnO2 4AI 3Mn 2Al203 3 H2S SO2 S H2O

Physical Chemistry
Atomic Structure68 The electron identified by quantum numbers n and 1 1 4 1 1 ii n 4 1 0 iii n 3 1 2 iv n 3 1 1 4 can be placed in order of increasing energy from the lowest to highest b ii iv i iii d iii i iv ii a iv ii iii i i iii ii iv Which represent the correct set up of the four quantum
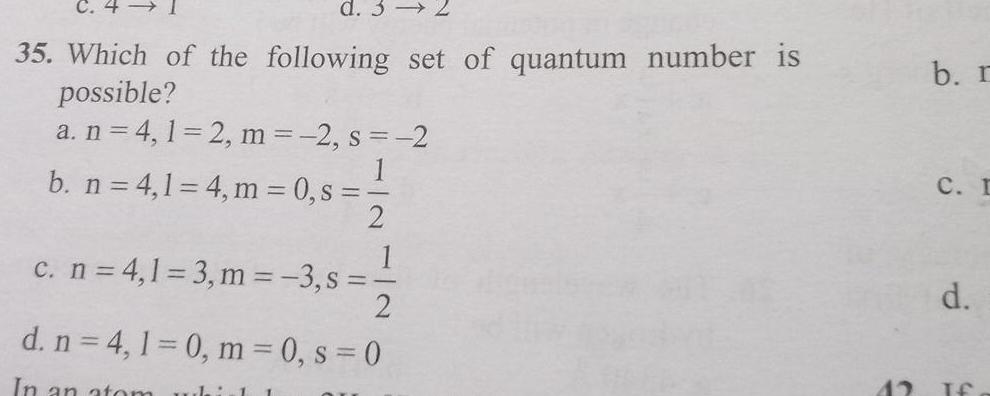
Physical Chemistry
Atomic Structured 3 35 Which of the following set of quantum number is possible a n 4 1 2 m 2 s 2 b n 4 1 4 m 0 s 2 1 c n 4 1 3 m 3 s 2 d n 4 1 0 m 0 s 0 In an atom whirl 1 42 b r C I d If

Physical Chemistry
Atomic StructureWhich one of the following is not the characteristic of Planck s quantum theory of radiation 1 The energy is not absorbed or emitted in whole number multiple of quantum 2 Radiation is associated with energy 3 Radiation energy is not emitted or absorbed continously but in the form of small packets called quan 4 This magnitude of energy associated with a quantum is proportional to the frequency Calculate the energy of a photon of sodium light of wave length 5 826 10 16 m in Jules

Physical Chemistry
Atomic StructureIn ncert it is written that nitrogen has higher atomic radius in comparison to oxygen and oxygen has higher atomic radius than fluori ne but in byjus video lessons its taught that oxygen fluorine nitrogen what to follow ple ase help

Physical Chemistry
Atomic StructureIf 10 17 of light energy is needed by the interior of human eye to see an object The number of photons of green light 1 550 nm needed to see the object are 1 27 2 28 3 29 4 30 Which of the following statements is false 1 The energy of red photon is more than the energy of violet photon 2 The momentum of photon is inversely proportional to its wave length 3 The energy of a photon is inversely proportional to its wave length

Physical Chemistry
Atomic StructureHow many photons of light having a wavelength of 5000 are necessary to provide 1 joule of energy 1 2 8 10 8 photons 10 7 photons 10 4 photons 3 2 5 1018 photons 2 2 5 4 2 6 RT Bohr Model The ionization energy of Het is 19 6 x 10 18 J atom The energy of the first stationary state of Li 2 will be 1 84 2 x 10 18 J atom 2 44 10 x 10 18 J atom

Physical Chemistry
Atomic StructureWhich of the following statements is false 1 The energy of red photon is more than the energy of violet photon 2 The momentum of photon is inversely proportional to its wave length 3 The energy of a photon is inversely proportional to its wave length 4 The particle nature of electromagnetic radiations is able to explain the photoelectric effect Light of wavelength I falls on metal having work function hc l Photoelectric effect will take place onl 21 COLL 21

Physical Chemistry
Atomic StructureAn atom has a mass of 0 02 kg and uncertainty in its velocity is 9 218 x 10 6 m s then uncertainty in position is h 6 626 10 34 Js A 2 86 1028 m C 1 5 10 27 m B 2 86 10 32 cm D 3 9 10 0 m

Physical Chemistry
Atomic StructureCalculate the energy of a photon of sodium light of wave length 5 826 1 3 41 10 J 2 1 3 41 10 1 J 3 3 41 10 15 J 10 16 m in Jules Calculate the frequency of a photon of wavelength 4000 1 7 5 10 4 S 1 2 7 5 10 16 S 1 3 8 10 4 S 1 Calculate the wavelength of a photon having an energy of 2 electron volt 4 1 3 41 10 2 J 4 6 5 10 15 S 1

Physical Chemistry
Atomic Structurea 2 2 x 10 19J c 4 x 10 20J b 2 10 J d 2 10 20J 1 What is the maximum number of electrons which can accomodated in principal quantum number 4 a 16 b 18 c 22 d 32

Physical Chemistry
Atomic Structureonly 6 In which transition one quantum of e emitted a n 4 n 2 b n 3 n 1 d All of these c n 2 n 1 7 Last line of Lyman series for H

Physical Chemistry
Atomic Structure18 If AE is the energy emitted in eV when an electronic transition occurs from higher energy level to lower energy level in H atom the of the line produced is approximately equal to b a C 12375 A AE 13600 A AE d 19800 A AE 21800 A AE

Physical Chemistry
Atomic Structurea 2 4 x 1025 Hz c 1 6 x 1015 Hz 5 The total number of orbitals in the principal shell of He Rhc where R is Rydberg 4 b 2 4 x 1016 Hz d 8 x 10 5 Hz that has energy equal to constant a 4 b 8 c 16 d 32

Physical Chemistry
Atomic StructureA 1 kW radio transmitter operates at a frequency of 880 Hz How many photons per second does it emit 1 1 71 x 10 2 1 71 x 1033 3 6 02 x 1023 4 2 85 x 1026 A bulb of 40 W is producing a light of wavelength 620 nm with 80 of efficiency then the number of photons emitted by the bulb in 20 seconds are 1eV 1 6 x 10 19 J hc 12400 eV 1 2 x 10 8 2 1018 3 1021 4 2 x 10 1

Physical Chemistry
Atomic StructureMaxwell said metal continuously absorb light or heat and emits continuously electron as light and this light is one colour but plank opposite to Maxwell plank said light is different colour because metal discontinuously absorb or emit light or heat 1 19 PM

Physical Chemistry
Atomic Structure1 The value of the spin only magnetic moment for one of the following configurations is 2 84 B M The correct one is a d in strong field ligand b d in weak field ligand c d in weak as well as in strong field ligand d 5 in strong field ligand

Physical Chemistry
Atomic Structure49 eV me quantum of energy is 3 n 1 of these or H atom has wavelength er series has wavelength d 12 The ionisation energy of H atom is x J atom The wavelength of first Balmer line for He ion is 5x a C 13 An 36 2 2 2mc22222 h 22 2 2 5x 36 hc b d 5x 9 9hc 5x

Physical Chemistry
Atomic Structure4 2 55 The maximum and minimum number of electrons in Co having 2 s 1 1 7 0 2 2 5 3 5 2 4 Can t be determined in its ground state will be 4 2 55 2 5 1 7 0 2 2 5 3 5 2 4 fruita WHE

Physical Chemistry
Atomic StructureThe energy required to break one mole of Cl Cl bonds in Cl is 242 kJ mol The longest wavelength of light capable of breaking a single C1 Cl bond is 1 23 c 3 108 m s and NA 6 02 x 10 mol x AIEEE 2010 DUMET 2010 A 494 nm C 640 nm B D 594 nm 700 nm

Physical Chemistry
Atomic StructureAccording to Heisenberg s principle the product of uncertainties and velocities for an 9 1 10 31 kg is A B uncertainty in position electron of mass 22 8 x 10 m s 2 1 23 8 x 105 m s 25 8 x 10 m s 2 768 x 10 6 2 1

Physical Chemistry
Atomic StructureThe uncertainties in the velocities of two particles A and B are 0 05 and 0 02 m s respectively The mass of B is five times to tha of the mass of A What is the ratio o uncertainties in their positions A 2 B 0 25 C 4 D 1 AXA AXB

Physical Chemistry
Atomic Structure2 Photoelectric emission is observed from a metal surface with incident frequencies v v where v v If the kinetic energies of the photoelectrons emitted in the two cases are in the ratio 2 1 then the threshold frequency v of the metal is a 2v V V V 2 C b 2v V d v V a 1 33 How Peri valu a C

Physical Chemistry
Atomic StructureThe velocity of particle A is 0 1m sec and that of particle B is 0 05m sec If the mass of particle B is 5 times that of particle A then the ratio of de broglie wavelength associated with particle A and B a 2 5 c 6 4 b 3 4 d 5 2

Physical Chemistry
Atomic Structure40 Which of the following complexes exhibits the highest paramagnetic behaviour Where gly glycine en ethylenediamine and bpy bipyridyl moities At No of Ti 22 V 23 Fe 26 Co 27 AIPMT Prelims 2008 1 Ti NH 3 Fe en bny NH 12 4 Colox OHLE 2 V gly OH NH3

Physical Chemistry
Atomic Structure151 ion Ma The number the number of electrons with m 0 s In an atom having 2K 8L 8M and 2N electrons are 1 6 3 8 2 2 4 16 of electrons in the M shell of the

Physical Chemistry
Atomic Structureb 24 eV d 216 eV wavelength of an electron moving in a s radius of the orbit is given by 2 d 2 2T 41 3 t light gives off yellow light that has a C d 4 2 1 1 2 9 Which of the following statements is are correct for electron having quantum numbers n 4 m 22 a The value of l may be 2 l b The value of may be 3 c The value of s may be 1 2 d All of these 10 in orbit angular momentum when an ol th CC F a 16 N S e 2 M

Physical Chemistry
Atomic Structureeet light gives off yellow light that has a 600 nm Then oton 12400eV A 0 A this light is 7x 10 s this light is 5 x 10 s ton is approximately 2 07 eV c avelength 310 pm is 10 Change in orbit angular momentum when an electro makes a transition corresponding to 3rd line Balmer series in Li ion is a h 2 C 2h b 3h 4h 2 2 11 An electron in a hydrogen d 2 0

Physical Chemistry
Atomic StructureA first line of Lymen series C last line of Paschen series B first line of Balmer series D last line of Bracket series Ratio of velocity of electron in 5th excited state and 3rd energy level for Het atom is A 9

Physical Chemistry
Atomic StructureWhich one is the wrong statement a The uncertainty principle is AEX At h 4T b Half filled and fully filled orbitals have greater stability due to greater exchange energy greater symmetry and more balanced arrangement c The energy of 2s orbital is less than the energy of 2p orbital in case of hydrogen like atoms d de Broglie s wavelength h is given by my m mass of the particle v group velocity of the particle where

Physical Chemistry
Atomic StructureSir why not answer should be option 4 correct 45 180 The Bohr model for the spectra of a H atom Will be applicable to hydrogen in the molecular form Will not be applicable as it is for a He atom Is valid at room temperature only Predicts continuous as well as discrete

Physical Chemistry
Atomic Structureemitted out to the number of quanta absorbed A photon of 300 nm is absorbed by a gas and then re emits two photons One re remitted photon has wa length 500 nm Calculate energy of other photon re emitted out

Physical Chemistry
Atomic Structure8 The second order Bragg diffraction of X rays with 1 00 A from a set of parallel planes in a metal occurs at an angle 60 The distance between the scattering planes in the crystal is 1 2 00 A 2 1 00 A 3 0 575 A 4 1 15 A

Physical Chemistry
Atomic StructureThe magnitude half of the orbital angular momentum vector of an electron in an orbital is expressed as Vo Into how many components will the vector split if a magnetic field is applied on it Rate this question Your Answer 2

Physical Chemistry
Atomic Structurea Find the location of elements with Z 12 16 8 18 b Find the elements in the above se t that are members of the 1 same grou p and 2 same period c Write the electrnic configuration o f an element X with atomic number 7 P redict the formula of its oxide Is it a m etal or a non metal

Physical Chemistry
Atomic Structure49 The atomic number of an element is 17 The number orbitals containing electron pairs in the valency shell is a 8 93 of the d 6 alomo

Physical Chemistry
Atomic Structurec Isol 179 Photoelectric emission is observed from a surface for frequency v and v2 of the incident radiation V V If the maximum kinetic energies of the photoelectrons in the two cases are in the ratio 1 k then the threshold frequency Vo is given by V2 V1 k 1 3 a c kv 2 V1 k 1 kv1 V2 k 1 14 d 2 V2 V1 k orhitals of element with ato

Physical Chemistry
Atomic StructureThe radial part of schrodinger wave equation for hydrogen atom is 1 0 2 y r 16 4a 2 1 0 o 80 12 e Where a constant o 2r na n principle quantum number Select the correct statements A Distance of nearest radial node from the nucleus is 2a B Distance of farthest radial node from the nucleus is 12a C Number of maxima in the curve y r vs r are 4 n r is for 4n orbital

Physical Chemistry
Atomic StructureUsing arbitrary energy units we can calculate that 864 arbitrary units a u are required to transfer an electron in hydrogen atom from the most stable Bohr s orbit to the largest distance from the nucleus n E 0 n 1 E 864 Arbitrary units The energy required to transfer the electron from third Bohr s orbit to the orbit n will be 1 96 Arbitrary units 2 192 Arbitrary units 3 288 Arbitrary units 4 384 Arbitrary units

Physical Chemistry
Atomic StructureA glow worm of mass 5 0 g emits red light 650 nm with a power of 0 1 W entirely in the backward direction What velocity would it accelerate to after 10 years if released in free space and assumed to be alive 1 9 14 ms 1 2 28 08 ms 1 3 11 05 ms 1 4 21 04 ms 1

Physical Chemistry
Atomic StructureSelect INCORRECT statement about the following molecular orbital O It is a bonding molecular orbital It is formed by overlapping of lobes of p and d2 which are in same phase Two atoms approach along z axis in order to form bond O Centre of symmetry is present in it

Physical Chemistry
Atomic StructureThe average atomic weight of copper which has two naturally occurring isotopes is 63 5 One of the isotopes has an atomic weight of 62 0 amu a constitutes 69 1 of the copper isotopes The other isotope has an abundance of 30 9 The atomic weight amu of the second isotope is 5 X amu

Physical Chemistry
Atomic StructureHelium atom can be excited to 1s 2p configuration by light of 58 44 nm The lowest excited state with configuration 1s 2s lies 4857 cm1 1 A below the 1s 2p state What would be the average He H bond energy so that HeH2 could form non endothermically from the lowest excited singlet state of helium Neglect any difference between AG and AH and take AH as 218 kJ mol 1 1614 kJ mol 1 2 1212 kJ mol 1 3 912 kJ mol 1 4 1511 kJ mol 1

Physical Chemistry
Atomic Structuremechanism catalyst following step s is are not involved in the of adsorption theory of heterogeneous 1 Diffusion of reactants to the surface of the catalyst ii Sorption of reactant molecules on the surface of the catalyst iii Occurrence of chemical reaction on the catalyst s surface through formation of an intermediate iv Desorption of reaction products from the catalyst s surface v Diffusion of reaction products away from the catalyst s surface a i only fo ii only b ii and iv d i ii and v

Physical Chemistry
Atomic Structure4 V2 V3 79 Consider the following statements 2 v A Electron density in XY Plane in 3dx y is zero B Electron density in XY plane in 3d orbital is zero C 2s orbital has only one spherical node D For 2P orbital YZ is the nodal plane 1 B and C 2 A B C and D 3 Only B 4 Only C Which is not the characteristic he V3 3 4v V 4 V V3 79 fuffed et for foru A 3d B 3d X C 2sha ya saa t D 2P fr YZ 1 BC 2 A B CD 3 B 4 C